Cancer stem cell-immune cell crosstalk in breast tumor microenvironment: a determinant of therapeutic facet
- PMID: 38090567
- PMCID: PMC10711058
- DOI: 10.3389/fimmu.2023.1245421
Cancer stem cell-immune cell crosstalk in breast tumor microenvironment: a determinant of therapeutic facet
Abstract
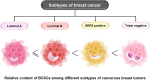
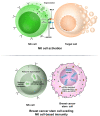
Breast cancer (BC) is globally one of the leading killers among women. Within a breast tumor, a minor population of transformed cells accountable for drug resistance, survival, and metastasis is known as breast cancer stem cells (BCSCs). Several experimental lines of evidence have indicated that BCSCs influence the functionality of immune cells. They evade immune surveillance by altering the characteristics of immune cells and modulate the tumor landscape to an immune-suppressive type. They are proficient in switching from a quiescent phase (slowly cycling) to an actively proliferating phenotype with a high degree of plasticity. This review confers the relevance and impact of crosstalk between immune cells and BCSCs as a fate determinant for BC prognosis. It also focuses on current strategies for targeting these aberrant BCSCs that could open avenues for the treatment of breast carcinoma.
Keywords: adaptive immune cells; breast cancer (BC); breast cancer stem cells (BCSCs); innate immune cells; tumor microenvironment (TME).
Copyright © 2023 Guha, Goswami, Sultana, Ganguly, Choudhury, Chakravarti, Bhuniya, Sarkar, Bera, Dhar, Das, Das, Baral, Bose and Banerjee.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures










References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

