DIALing-up the preclinical characterization of gene-modified adoptive cellular immunotherapies
- PMID: 38090585
- PMCID: PMC10713823
- DOI: 10.3389/fimmu.2023.1264882
DIALing-up the preclinical characterization of gene-modified adoptive cellular immunotherapies
Abstract
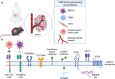
The preclinical characterization of gene modified adoptive cellular immunotherapy candidates for clinical development often requires the use of mouse models. Gene-modified lymphocytes (GML) incorporating chimeric antigen receptors (CAR) and T-cell receptors (TCR) into immune effector cells require in vivo characterization of biological activity, mechanism of action, and preclinical safety. Typically, this characterization involves the assessment of dose-dependent, on-target, on-tumor activity in severely immunocompromised mice. While suitable for the purpose of evaluating T cell-expressed transgene function in a living host, this approach falls short in translating cellular therapy efficacy, safety, and persistence from preclinical models to humans. To comprehensively characterize cell therapy products in mice, we have developed a framework called "DIAL". This framework aims to enable an end-to-end understanding of genetically engineered cellular immunotherapies in vivo, from infusion to tumor clearance and long-term immunosurveillance. The acronym DIAL stands for Distribution, Infiltration, Accumulation, and Longevity, compartmentalizing the systemic attributes of gene-modified cellular therapy and providing a platform for optimization with the ultimate goal of improving therapeutic efficacy. This review will discuss both existent and emerging examples of DIAL characterization in mouse models, as well as opportunities for future development and optimization.
Keywords: CAR-T cells; adoptive cell therapy; animal models; immunotherapy; tumor microenvironment.
Copyright © 2023 Giardino Torchia and Moody.
Conflict of interest statement
All authors are employees of AstraZeneca, PLC and have financial interest in the company.
Figures




References
-
- Rosenberg SA, Aebersold P, Cornetta K, Kasid A, Morgan RA, Moen R, et al. . Gene transfer into humans–immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med (1990) 323(9):570–8. doi: 10.1056/NEJM199008303230904 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

