The Effect of Corticosteroids on Post-Covid-19 Smell Loss: A Meta-Analysis
- PMID: 38090618
- PMCID: PMC10712548
- DOI: 10.22038/IJORL.2023.72451.3456
The Effect of Corticosteroids on Post-Covid-19 Smell Loss: A Meta-Analysis
Abstract
Introduction: The rate of olfactory loss related to COVID-19 was reported between 4-89 percent. There is no approved treatment for patients who experience anosmia after the mentioned infection. This systematic review aimed to assess the therapeutic effects of corticosteroids on anosmia in COVID-19 patients.
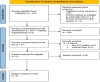
Materials and methods: Databases including PubMed, ISI Web of Sciences, Scopus, and Cochrane Library. Databases were searched up to September 2022 to find out randomized controlled trials that assessed the effect of corticosteroids on post-COVID anosmia/hyposmia. Only studies published in the English language were entered in this review.
Results: Among the six relevant trials with a total population of 712, one study administered the combination therapy of both systemic and nasal corticosteroids, while others used intranasal corticosteroids. No significant difference was observed between the intervention (IG) and control (CG) groups in terms of duration of improvement from anosmia (mean difference:-1.799). The pooled effect of self-rating olfactory scores was assessed at 2 weeks and at the end point of the studies which revealed no significant effect in favor of the IG (pooled effect in 2 weeks: 0.739; in the endpoint: 1.32). The objective evaluation with different tools indicated that IG obtained higher scores at the endpoint of treatment. The pooled results showed that the number of patients who recovered from anosmia is higher in IG compared to CG (Odds Ratio: 1.719).
Conclusion: It appears that the duration of corticosteroid therapy more than two weeks may be a considerable effect on the recovery of smell dysfunction in COVID-19 patients.
Keywords: Anosmia; COVID-19; Corticosteroid; Hyposmia; Olfactory.
Figures



Similar articles
-
Intranasal Corticosteroid Treatment on Recovery of Long-Term Olfactory Dysfunction Due to COVID-19.Laryngoscope. 2022 Nov;132(11):2209-2216. doi: 10.1002/lary.30353. Epub 2022 Aug 25. Laryngoscope. 2022. PMID: 36054369 Free PMC article. Clinical Trial.
-
Effect of any form of steroids in comparison with that of other medications on the duration of olfactory dysfunction in patients with COVID-19: A systematic review of randomized trials and quasi-experimental studies.PLoS One. 2023 Aug 2;18(8):e0288285. doi: 10.1371/journal.pone.0288285. eCollection 2023. PLoS One. 2023. PMID: 37531338 Free PMC article.
-
Corticosteroid nasal spray for recovery of smell sensation in COVID-19 patients: A randomized controlled trial.Am J Otolaryngol. 2021 Mar-Apr;42(2):102884. doi: 10.1016/j.amjoto.2020.102884. Epub 2021 Jan 4. Am J Otolaryngol. 2021. PMID: 33429174 Free PMC article. Clinical Trial.
-
Efficacy of topical steroids for the treatment of olfactory disorders caused by COVID-19: A systematic review and meta-analysis.Clin Otolaryngol. 2022 Jul;47(4):509-515. doi: 10.1111/coa.13933. Epub 2022 Apr 6. Clin Otolaryngol. 2022. PMID: 35352483 Free PMC article.
-
Corticosteroid nasal irrigation as early treatment of olfactory dysfunction in COVID-19: A prospective randomised controlled trial.Clin Otolaryngol. 2023 Mar;48(2):182-190. doi: 10.1111/coa.14004. Epub 2022 Nov 14. Clin Otolaryngol. 2023. PMID: 36336851 Free PMC article. Clinical Trial.
References
-
- WHO. https://covid19.who.int /
LinkOut - more resources
Full Text Sources
