Safety of AZD1222 COVID-19 vaccine and low Incidence of SARS-CoV-2 infection in Botswana following ChAdOx1(AZD1222) vaccination: A single-arm open-label interventional study - final study results
- PMID: 38090729
- PMCID: PMC10714336
- DOI: 10.1016/j.ijregi.2023.11.002
Safety of AZD1222 COVID-19 vaccine and low Incidence of SARS-CoV-2 infection in Botswana following ChAdOx1(AZD1222) vaccination: A single-arm open-label interventional study - final study results
Abstract
Objectives: We report the final analysis of the single-arm open-label study evaluating the safety and COVID-19 incidence after AZD1222 vaccination in Botswana conducted between September 2021 and August 2022.
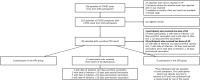
Methods: The study included three groups of adults (>18 years), homologous AZD1222 primary series and booster (AZ2), heterologous primary series with one dose AZD1222, and AZD1222 booster (HPS), and primary series other than AZD1222 and AZD1222 booster (OPS). We compared the incidence of AEs in participants with and without prior COVID-19 infection using an exact test for rate ratios.
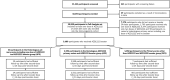
Results: Among 10,894 participants, 9192 (84.4%) were enrolled at first vaccine dose, 521 (4.8%) at second vaccine, and 1181 (10.8%) at the booster vaccine. Of 10,855 included in the full analysis set, 1700 received one dose of AZD1222; 5377 received two doses; 98 received a heterologous series including one AZD1222 and a booster; 30 in the HPS group; 1058 in the OPS group; and 2592 in the AZ2 group. No laboratory-confirmed COVID-19 hospitalizations or deaths were reported. The incidence of laboratory-confirmed symptomatic COVID infection for the AZ2 group was 6.22 (95% confidence interval: 2.51-12.78) per 1000 participant-years (1000-PY) and 3.5 (95% confidence interval: 0.42-12.57) per 1000-PY for AZ2+booster group. Most adverse events were mild, with higher incidence in participants with prior COVID-19 infection. Individuals with prior COVID-19 exposure exhibited higher binding antibody responses. No differences in outcomes were observed by HIV status.
Conclusion: AZD1222 is safe, effective, and immunogenic for people living with and without HIV.
Keywords: AZD 1222; Antibody responses; AstraZeneca; Botswana; COVID-19 vaccination.
© 2023 The Author(s).
Conflict of interest statement
ST and PG are employees of, and hold or may hold stock in, AstraZeneca. AW is an employee of X4 Group contracted to AstraZeneca for this work. LC is an employee of SRG Recruitment contracted to AstraZeneca for this work. All other authors declare that they have no conflict of interest.
Figures
References
-
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. - DOI - PMC - PubMed
-
- Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. - DOI - PMC - PubMed
-
- UNAIDS . Global Aids Monitoring; Botswana: 2020. Country progress report-Botswana.
-
- Gaolathe T, Wirth KE, Holme MP, Makhema J, Moyo S, Chakalisa U, et al. Botswana's progress toward achieving the 2020 UNAIDS 90-90-90 antiretroviral therapy and virological suppression goals: a population-based survey. Lancet HIV. 2016;3:e221–e230. doi: 10.1016/S2352-3018(16)00037-0. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous