Portable hypothermic oxygenated machine perfusion for organ preservation in liver transplantation: A randomized, open-label, clinical trial
- PMID: 38090880
- PMCID: PMC11019979
- DOI: 10.1097/HEP.0000000000000715
Portable hypothermic oxygenated machine perfusion for organ preservation in liver transplantation: A randomized, open-label, clinical trial
Abstract
Background and aims: In liver transplantation, cold preservation induces ischemia, resulting in significant reperfusion injury. Hypothermic oxygenated machine perfusion (HMP-O 2 ) has shown benefits compared to static cold storage (SCS) by limiting ischemia-reperfusion injury. This study reports outcomes using a novel portable HMP-O 2 device in the first US randomized control trial.
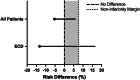
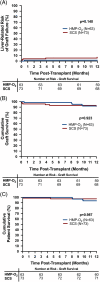
Approach and results: The PILOT trial (NCT03484455) was a multicenter, randomized, open-label, noninferiority trial, with participants randomized to HMP-O 2 or SCS. HMP-O 2 livers were preserved using the Lifeport Liver Transporter and Vasosol perfusion solution. The primary outcome was early allograft dysfunction. Noninferiority margin was 7.5%. From April 3, 2019, to July 12, 2022, 179 patients were randomized to HMP-O 2 (n=90) or SCS (n=89). The per-protocol cohort included 63 HMP-O 2 and 73 SCS. Early allograft dysfunction occurred in 11.1% HMP-O 2 (N=7) and 16.4% SCS (N=12). The risk difference between HMP-O 2 and SCS was -5.33% (one-sided 95% upper confidence limit of 5.81%), establishing noninferiority. The risk of graft failure as predicted by Liver Graft Assessment Following Transplant score at seven days (L-GrAFT 7 ) was lower with HMP-O 2 [median (IQR) 3.4% (2.4-6.5) vs. 4.5% (2.9-9.4), p =0.024]. Primary nonfunction occurred in 2.2% of all SCS (n=3, p =0.10). Biliary strictures occurred in 16.4% SCS (n=12) and 6.3% (n=4) HMP-O 2 ( p =0.18). Nonanastomotic biliary strictures occurred only in SCS (n=4).
Conclusions: HMP-O 2 demonstrates safety and noninferior efficacy for liver graft preservation in comparison to SCS. Early allograft failure by L-GrAFT 7 was lower in HMP-O 2 , suggesting improved early clinical function. Recipients of HMP-O 2 livers also demonstrated a lower incidence of primary nonfunction and biliary strictures, although this difference did not reach significance.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Guergana G. Panayotova received travel grants from Organ Recovery Systems. Keri E. Lunsford consults for GATT Technologies. She owns stock in Henry Schein, Incyte, Intellia Therapeutics, Johnson & Johnson, ROM Tech, and Tandem Diabetes. R. Cutler Quillin, III consults and received travel grants for Ethicon. Grace S. Lee-Riddle consults for EBSCO-Dynamed. Daniel Borja-Cacho received travel grants from Organ Recovery Systems and Xvivo Perfusion. Shimul A. Shah consults and advises Genentech. He received grants from CareDx and Organ Recovery Systems. James V. Guarrera consults and received travel grants from GATT Technologies and Organ Recovery Systems. The remaining authors have no conflicts to report.
Figures




References
-
- Kwong AJ, Ebel NH, Kim WR, Lake JR, Smith JM, Schladt DP, et al. . OPTN/SRTR 2020 annual data report: Liver. Am J Transplant. 2022;22(suppl 2):204–309. - PubMed
-
- Sher L, Quintini C, Fayek SA, Abt P, Lo M, Yuk P, et al. . Attitudes and barriers to the use of donation after cardiac death livers: Comparison of a United States transplant center survey to the united network for organ sharing data. Liver Transpl. 2017;23:1372–83. - PubMed
-
- Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, et al. . Projected U.S. State-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440–50. - PubMed
-
- Panayotova GG, Rosado J, Paterno F, Deo D, Dikdan G, McCarty MA, et al. . Novel oxygenation technique for hypothermic machine perfusion of liver grafts: Validation in porcine Donation after Cardiac Death liver model. Am J Surg. 2020;220:1270–7. - PubMed
-
- Guarrera JV, Henry SD, Samstein B, Reznik E, Musat C, Lukose TI, et al. . Hypothermic machine preservation facilitates successful transplantation of “orphan” extended criteria donor livers. Am J Transplant. 2015;15:161–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

