Mineralocorticoid Receptor Antagonism by Eplerenone and Arterial Inflammation in HIV: The MIRABELLA HIV Study
- PMID: 38090987
- PMCID: PMC10719827
- DOI: 10.1001/jamacardio.2023.4578
Mineralocorticoid Receptor Antagonism by Eplerenone and Arterial Inflammation in HIV: The MIRABELLA HIV Study
Abstract
Importance: The risk for atherosclerotic disease is increased 1.5- to 2.0-fold among persons with HIV (PWH). Increased activation of the renin-angiotensin-aldosterone system may contribute to increased arterial inflammation in this population.
Objective: To determine the effects of eplerenone on arterial inflammation among well-treated PWH without known cardiovascular disease (CVD).
Design, setting, and participants: Well-treated PWH who participated in the double-blinded, placebo-controlled, Mineralocorticoid Receptor Antagonism for Cardiovascular Health in HIV (MIRACLE HIV) study between February 2017 and March 2022 assessing the effects of eplerenone on myocardial perfusion were invited to participate in the Mineralocorticoid Receptor Antagonism By Eplerenone to Lower Arterial Inflammation in HIV (MIRABELLA) substudy if there was no current statin use. Participants were enrolled in the MIRABELLA study and underwent additional 18F-fludeoxyglucose-positron emission tomography/computed tomography (18F-FDG PET/CT) imaging of the aorta and carotid arteries to assess arterial inflammation over 12 months of treatment with eplerenone vs placebo.
Interventions: Eplerenone, 50 mg, twice a day vs identical placebo.
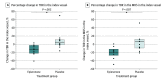
Main outcomes and measures: The primary outcome was change in target to background ratio (TBR), a measure of arterial wall inflammation, in the index vessel after 12 months of treatment. The index vessel was defined as the vessel (aorta, left carotid artery, or right carotid artery) with the highest TBR at baseline in each participant.
Results: A total of 26 participants (mean [SD] age, 54 [7] years; 18 male [69%]) were enrolled in the study. Treatment groups (eplerenone, 13 vs placebo, 13) were of similar age, sex, and body mass index. Eplerenone was associated with a reduction in TBR of the primary end point, the index vessel (eplerenone vs placebo: model treatment effect, -0.31; 95% CI, -0.50 to -0.11; P = .006; percentage change, -12.4% [IQR, -21.9% to -2.6%] vs 5.1% [IQR, -1.6% to 11.0%]; P = .003). We further observed a significant reduction of the TBR of the most diseased segment (MDS) of the index vessel (eplerenone vs placebo: -19.1% [IQR, -27.0% to -11.9%] vs 6.8% [IQR, -9.1% to 12.1%]; P = .007). A similar result was seen assessing the index vessel of the carotids (eplerenone vs placebo: -10.0% [IQR, -21.8% to 3.6%] vs 9.7% [IQR, -9.8% to 15.9%]; P = .046). Reduction in the TBR of MDS of the index vessel on 18F-FDG PET/CT correlated with improvement in the stress myocardial blood flow on cardiac magnetic resonance imaging (Spearman ρ = -0.67; P = .01).
Conclusion and relevance: In this small randomized clinical trial, eplerenone was associated with reduction in arterial inflammation among well-treated PWH without known CVD. In addition, reductions in arterial inflammation as measured by 18F-FDG PET/CT were related to improvements in stress myocardial perfusion. Further larger studies should explore whether eplerenone is a potential treatment strategy for inflammatory-mediated CVD in PWH.
Trial registration: ClinicalTrials.gov Identifier: NCT02740179.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

