The mitochondrial ATP synthase is a negative regulator of the mitochondrial permeability transition pore
- PMID: 38091291
- PMCID: PMC10743364
- DOI: 10.1073/pnas.2303713120
The mitochondrial ATP synthase is a negative regulator of the mitochondrial permeability transition pore
Abstract
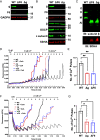
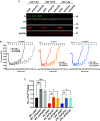
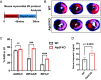
The mitochondrial permeability transition pore (mPTP) is a channel in the inner mitochondrial membrane whose sustained opening in response to elevated mitochondrial matrix Ca2+ concentrations triggers necrotic cell death. The molecular identity of mPTP is unknown. One proposed candidate is the mitochondrial ATP synthase, whose canonical function is to generate most ATP in multicellular organisms. Here, we present mitochondrial, cellular, and in vivo evidence that, rather than serving as mPTP, the mitochondrial ATP synthase inhibits this pore. Our studies confirm previous work showing persistence of mPTP in HAP1 cell lines lacking an assembled mitochondrial ATP synthase. Unexpectedly, however, we observe that Ca2+-induced pore opening is markedly sensitized by loss of the mitochondrial ATP synthase. Further, mPTP opening in cells lacking the mitochondrial ATP synthase is desensitized by pharmacological inhibition and genetic depletion of the mitochondrial cis-trans prolyl isomerase cyclophilin D as in wild-type cells, indicating that cyclophilin D can modulate mPTP through substrates other than subunits in the assembled mitochondrial ATP synthase. Mitoplast patch clamping studies showed that mPTP channel conductance was unaffected by loss of the mitochondrial ATP synthase but still blocked by cyclophilin D inhibition. Cardiac mitochondria from mice whose heart muscle cells we engineered deficient in the mitochondrial ATP synthase also demonstrate sensitization of Ca2+-induced mPTP opening and desensitization by cyclophilin D inhibition. Further, these mice exhibit strikingly larger myocardial infarctions when challenged with ischemia/reperfusion in vivo. We conclude that the mitochondrial ATP synthase does not function as mPTP and instead negatively regulates this pore.
Keywords: mitochondrial ATP synthase; mitochondrial permeability transition pore; necrosis.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Raaflaub J., Die schwellung isolierter leberzell mitochondrien und ihre physikalisch beeinflußarkeit. Helv. Physiol. Pharmacol. Acta. 11, 142–156 (1953). - PubMed
-
- Hunter D. R., Haworth R. A., Southard J. H., Relationship between configuration, function, and permeability in calcium-treated mitochondria. J. Biol. Chem. 251, 5069–5077 (1976). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

