An inflammation resolution-promoting intervention prevents atrial fibrillation caused by left ventricular dysfunction
- PMID: 38091977
- PMCID: PMC10981525
- DOI: 10.1093/cvr/cvad175
An inflammation resolution-promoting intervention prevents atrial fibrillation caused by left ventricular dysfunction
Abstract
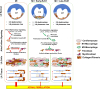
Aims: Recent studies suggest that bioactive mediators called resolvins promote an active resolution of inflammation. Inflammatory signalling is involved in the development of the substrate for atrial fibrillation (AF). The aim of this study is to evaluate the effects of resolvin-D1 on atrial arrhythmogenic remodelling resulting from left ventricular (LV) dysfunction induced by myocardial infarction (MI) in rats.
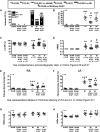
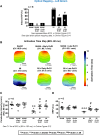
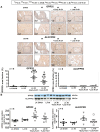
Methods and results: MI was produced by left anterior descending coronary artery ligation. Intervention groups received daily intraperitoneal resolvin-D1, beginning before MI surgery (early-RvD1) or Day 7 post-MI (late-RvD1) and continued until Day 21 post-MI. AF vulnerability was evaluated by performing an electrophysiological study. Atrial conduction was analysed by using optical mapping. Fibrosis was quantified by Masson's trichrome staining and gene expression by quantitative polymerase chain reaction and RNA sequencing. Investigators were blinded to group identity. Early-RvD1 significantly reduced MI size (17 ± 6%, vs. 39 ± 6% in vehicle-MI) and preserved LV ejection fraction; these were unaffected by late-RvD1. Transoesophageal pacing induced atrial tachyarrhythmia in 2/18 (11%) sham-operated rats, vs. 18/18 (100%) MI-only rats, in 5/18 (28%, P < 0.001 vs. MI) early-RvD1 MI rats, and in 7/12 (58%, P < 0.01) late-RvD1 MI rats. Atrial conduction velocity significantly decreased post-MI, an effect suppressed by RvD1 treatment. Both early-RvD1 and late-RvD1 limited MI-induced atrial fibrosis and prevented MI-induced increases in the atrial expression of inflammation-related and fibrosis-related biomarkers and pathways.
Conclusions: RvD1 suppressed MI-related atrial arrhythmogenic remodelling. Early-RvD1 had MI sparing and atrial remodelling suppressant effects, whereas late-RvD1 attenuated atrial remodelling and AF promotion without ventricular protection, revealing atrial-protective actions unrelated to ventricular function changes. These results point to inflammation resolution-promoting compounds as novel cardio-protective interventions with a particular interest in attenuating AF substrate development.
Keywords: Atrial fibrillation; Electrophysiology; Fibrosis; Myocardial infarction; Resolvin.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest: None declared.
Figures




Comment in
-
A new year's resolution to resolve atrial fibrillation: Resolvin D1 emerges as a powerful target against post-MI atrial remodelling.Cardiovasc Res. 2024 Mar 30;120(4):329-330. doi: 10.1093/cvr/cvae039. Cardiovasc Res. 2024. PMID: 38387430 Free PMC article. No abstract available.
References
-
- Thihalolipavan S, Morin DP. Atrial fibrillation and congestive heart failure. Heart Fail Clin 2014;10:305–318. - PubMed
-
- Hiram R, Naud P, Xiong F, Al-U’Datt D, Algalarrondo V, Sirois MG, Tanguay JF, Tardif JC, Nattel S. Right atrial mechanisms of atrial fibrillation in a rat model of right heart disease. J Am Coll Cardiol 2019;74:1332–1347. - PubMed
-
- Harada M, Nattel S. Implications of inflammation and fibrosis in atrial fibrillation pathophysiology. Card Electrophysiol Clin 2021;13:25–35. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

