trans-Endothelial neutrophil migration activates bactericidal function via Piezo1 mechanosensing
- PMID: 38091995
- PMCID: PMC10872880
- DOI: 10.1016/j.immuni.2023.11.007
trans-Endothelial neutrophil migration activates bactericidal function via Piezo1 mechanosensing
Abstract
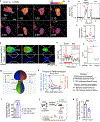
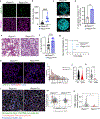
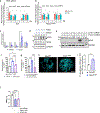
The regulation of polymorphonuclear leukocyte (PMN) function by mechanical forces encountered during their migration across restrictive endothelial cell junctions is not well understood. Using genetic, imaging, microfluidic, and in vivo approaches, we demonstrated that the mechanosensor Piezo1 in PMN plasmalemma induced spike-like Ca2+ signals during trans-endothelial migration. Mechanosensing increased the bactericidal function of PMN entering tissue. Mice in which Piezo1 in PMNs was genetically deleted were defective in clearing bacteria, and their lungs were predisposed to severe infection. Adoptive transfer of Piezo1-activated PMNs into the lungs of Pseudomonas aeruginosa-infected mice or exposing PMNs to defined mechanical forces in microfluidic systems improved bacterial clearance phenotype of PMNs. Piezo1 transduced the mechanical signals activated during transmigration to upregulate nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 4, crucial for the increased PMN bactericidal activity. Thus, Piezo1 mechanosensing of increased PMN tension, while traversing the narrow endothelial adherens junctions, is a central mechanism activating the host-defense function of transmigrating PMNs.
Keywords: Nox4; Piezo1; calcium signaling; mechanical signaling; neutrophil; phagocytosis; pneumonia.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



Comment in
-
The effect of transient, constricted migration on neutrophil intracellular bacteria-killing capability.Immunity. 2024 Aug 13;57(8):1713-1715. doi: 10.1016/j.immuni.2024.07.008. Immunity. 2024. PMID: 39142267 Free PMC article. No abstract available.
-
Challenging the role of a NOX4-Piezo1 axis in neutrophil bactericidal function.Immunity. 2024 Aug 13;57(8):1716-1718. doi: 10.1016/j.immuni.2024.07.012. Immunity. 2024. PMID: 39142268 Free PMC article. No abstract available.
-
Neutrophils meet their fate in endothelial adherens junctions.Immunity. 2024 Aug 13;57(8):1719-1720. doi: 10.1016/j.immuni.2024.07.015. Immunity. 2024. PMID: 39142269 Free PMC article. No abstract available.
References
-
- Dziarski R, Platt KA, Gelius E, Steiner H, and Gupta D (2003). Defect in neutrophil killing and increased susceptibility to infection with nonpathogenic gram-positive bacteria in peptidoglycan recognition protein-S (PGRP-S)-deficient mice. Blood 102, 689–697. 10.1182/blood-2002-12-3853. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

