Dabrafenib plus trametinib in unselected advanced BRAF V600-mut melanoma: a non-interventional, multicenter, prospective trial
- PMID: 38092013
- PMCID: PMC10906199
- DOI: 10.1097/CMR.0000000000000948
Dabrafenib plus trametinib in unselected advanced BRAF V600-mut melanoma: a non-interventional, multicenter, prospective trial
Abstract
Objective: The efficacy of combined BRAF and MEK inhibition for BRAF V600-mutant melanoma in a broad patient population, including subgroups excluded from phase 3 trials, remains unanswered. This noninterventional study (DATUM-NIS) assessed the real-world efficacy, safety and tolerability of dabrafenib plus trametinib in Austrian patients with unresectable/metastatic melanoma.
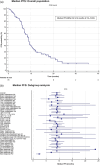
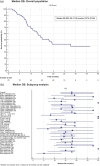
Methods: This multicenter, open-label, non-interventional, post-approval, observational study investigated the effectiveness of dabrafenib plus trametinib prescribed in day-to-day clinical practice to patients ( N = 79) with BRAF V600-mutant unresectable/metastatic melanoma with M1c disease (American Joint Committee on Cancer staging manual version 7), ECOG > 1, and elevated serum lactate dehydrogenase (LDH). The primary endpoint was 6-, 12- and 18-month progression-free survival (PFS) rates. Secondary endpoints were median PFS, disease control rate and overall survival (OS).
Results: The 6-, 12- and 18-month PFS rates were 76%, 30.6% and 16.2%, respectively. Subgroup analysis showed a significant PFS benefit in the absence of lung metastasis. The median PFS and OS were 9.1 (95% CI, 7.1-10.3) months and 17.9 (95% CI, 12.7-27.8) months, respectively. The 12- and 24-month OS rates were 62.7% and 26.8%, respectively. Subgroup analyses showed significant OS benefits in the absence of bone or lung metastasis and the presence of other metastases (excluding bone, lung, brain, liver and lymph nodes). Furthermore, S100 and Eastern Cooperative Oncology Group performance status (ECOG PS) showed a significant impact on survival. No new safety signals were observed.
Conclusion: Despite an unselected population of melanoma patients with higher M1c disease, ECOG PS > 1 and elevated LDH, this real-world study demonstrated comparable efficacy and safety with the pivotal phase 3 clinical trials for dabrafenib-trametinib.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- American Cancer Society. 5-Year relative survival rates for melanoma skin cancer. https://www.cancer.org/cancer/melanoma-skin-cancer/detection-diagnosis-s.... [Accessed 17 April 2021].
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. . Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–249. - PubMed
-
- International Agency for Research on Cancer. Austria fact sheet. https://gco.iarc.fr/today/data/factsheets/populations/40-austria-fact-sh.... [Accessed 17 April 2021].
-
- Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. . Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015; 372:30–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

