GDF15 linked to maternal risk of nausea and vomiting during pregnancy
- PMID: 38092039
- PMCID: PMC10808057
- DOI: 10.1038/s41586-023-06921-9
GDF15 linked to maternal risk of nausea and vomiting during pregnancy
Abstract
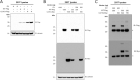
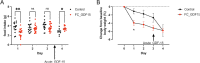
GDF15, a hormone acting on the brainstem, has been implicated in the nausea and vomiting of pregnancy, including its most severe form, hyperemesis gravidarum (HG), but a full mechanistic understanding is lacking1-4. Here we report that fetal production of GDF15 and maternal sensitivity to it both contribute substantially to the risk of HG. We confirmed that higher GDF15 levels in maternal blood are associated with vomiting in pregnancy and HG. Using mass spectrometry to detect a naturally labelled GDF15 variant, we demonstrate that the vast majority of GDF15 in the maternal plasma is derived from the feto-placental unit. By studying carriers of rare and common genetic variants, we found that low levels of GDF15 in the non-pregnant state increase the risk of developing HG. Conversely, women with β-thalassaemia, a condition in which GDF15 levels are chronically high5, report very low levels of nausea and vomiting of pregnancy. In mice, the acute food intake response to a bolus of GDF15 is influenced bi-directionally by prior levels of circulating GDF15 in a manner suggesting that this system is susceptible to desensitization. Our findings support a putative causal role for fetally derived GDF15 in the nausea and vomiting of human pregnancy, with maternal sensitivity, at least partly determined by prepregnancy exposure to the hormone, being a major influence on its severity. They also suggest mechanism-based approaches to the treatment and prevention of HG.
© 2023. The Author(s).
Conflict of interest statement
D.S.C.-J. reports non-financial support from Roche Diagnostics Ltd, outside the submitted work; G.C.S.S. reports personal fees and non-financial support from Roche Diagnostics Ltd, outside the submitted work; D.S.C.-J. and G.C.S.S. report grants from Sera Prognostics Inc, non-financial support from Illumina Inc, outside the submitted work. G.C.S.S. has been a paid consultant to GSK (preterm birth) and is a member of a Data Monitoring Committee for GSK trials of RSV vaccination in pregnancy. N.S. and P.W. have received grant funding from Roche Diagnostics paid to their institution for biomarker work inclusive of GDF15 measurements. J.R.B.P. is an employee and shareholder of Adrestia Therapeutics Ltd. K.M.G. is a paid consultant for BYOMass Inc. C.L.M. has received research funding and equipment at reduced cost from Dexcom Inc. G.T. is a full-time employee of Regeneron Genetics Center and receives salary, stock and stock options as compensation. F.M.G. has received research grant support from Eli-Lilly and AstraZeneca outside the scope of this current work. M.S.F. is a paid consultant for Materna Biosciences, Inc., NGM Biopharmaceuticals, and a Board member and Science Advisor for the Hyperemesis Education and Research Foundation. S.O. has undertaken remunerated consultancy work for Pfizer, Third Rock Ventures, AstraZeneca, NorthSea Therapeutics and Courage Therapeutics. Part of the work in this paper is the subject of a pending patent application relating to therapy for hyperemesis gravidarum filed by Cambridge Enterprise Limited (GB application No. 2304716.0; Inventor: Professor Stephen O’Rahilly). S.L. and N.R. are named creators on this patent. All other authors declare no competing interests.
Figures








References
MeSH terms
Substances
Grants and funding
- MR/M009041/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_00014/5/MRC_/Medical Research Council/United Kingdom
- G1001995/MRC_/Medical Research Council/United Kingdom
- R01 HG012133/HG/NHGRI NIH HHS/United States
- MC_UU_12015/2/MRC_/Medical Research Council/United Kingdom
- MC_UU_00014/1/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- MC_UU_00006/2/MRC_/Medical Research Council/United Kingdom
- MC_UU_00007/10/MRC_/Medical Research Council/United Kingdom
- R01 GM140287/GM/NIGMS NIH HHS/United States
- MC_UU_00014/3/MRC_/Medical Research Council/United Kingdom
- P01 CA196569/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

