Modelling post-implantation human development to yolk sac blood emergence
- PMID: 38092041
- PMCID: PMC10849971
- DOI: 10.1038/s41586-023-06914-8
Modelling post-implantation human development to yolk sac blood emergence
Abstract
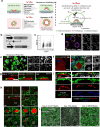
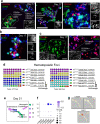
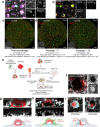
Implantation of the human embryo begins a critical developmental stage that comprises profound events including axis formation, gastrulation and the emergence of haematopoietic system1,2. Our mechanistic knowledge of this window of human life remains limited due to restricted access to in vivo samples for both technical and ethical reasons3-5. Stem cell models of human embryo have emerged to help unlock the mysteries of this stage6-16. Here we present a genetically inducible stem cell-derived embryoid model of early post-implantation human embryogenesis that captures the reciprocal codevelopment of embryonic tissue and the extra-embryonic endoderm and mesoderm niche with early haematopoiesis. This model is produced from induced pluripotent stem cells and shows unanticipated self-organizing cellular programmes similar to those that occur in embryogenesis, including the formation of amniotic cavity and bilaminar disc morphologies as well as the generation of an anterior hypoblast pole and posterior domain. The extra-embryonic layer in these embryoids lacks trophoblast and shows advanced multilineage yolk sac tissue-like morphogenesis that harbours a process similar to distinct waves of haematopoiesis, including the emergence of erythroid-, megakaryocyte-, myeloid- and lymphoid-like cells. This model presents an easy-to-use, high-throughput, reproducible and scalable platform to probe multifaceted aspects of human development and blood formation at the early post-implantation stage. It will provide a tractable human-based model for drug testing and disease modelling.
© 2023. The Author(s).
Conflict of interest statement
The University of Pittsburgh has a pending patent on behalf of the inventors J.H., R.S., S.K. and M.R.E., application no. PCT/US2023/067404, covering the derivation and generation of cell and extracellular products shown in this paper. All other authors declare no competing interests.
Figures














Update of
-
Modelling Human Post-Implantation Development via Extra-Embryonic Niche Engineering.bioRxiv [Preprint]. 2023 Jul 24:2023.06.15.545118. doi: 10.1101/2023.06.15.545118. bioRxiv. 2023. Update in: Nature. 2024 Feb;626(7998):367-376. doi: 10.1038/s41586-023-06914-8. PMID: 37398391 Free PMC article. Updated. Preprint.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

