The endoplasmic reticulum: Homeostasis and crosstalk in retinal health and disease
- PMID: 38092262
- PMCID: PMC11056313
- DOI: 10.1016/j.preteyeres.2023.101231
The endoplasmic reticulum: Homeostasis and crosstalk in retinal health and disease
Abstract
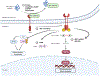
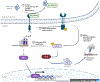
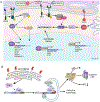
The endoplasmic reticulum (ER) is the largest intracellular organelle carrying out a broad range of important cellular functions including protein biosynthesis, folding, and trafficking, lipid and sterol biosynthesis, carbohydrate metabolism, and calcium storage and gated release. In addition, the ER makes close contact with multiple intracellular organelles such as mitochondria and the plasma membrane to actively regulate the biogenesis, remodeling, and function of these organelles. Therefore, maintaining a homeostatic and functional ER is critical for the survival and function of cells. This vital process is implemented through well-orchestrated signaling pathways of the unfolded protein response (UPR). The UPR is activated when misfolded or unfolded proteins accumulate in the ER, a condition known as ER stress, and functions to restore ER homeostasis thus promoting cell survival. However, prolonged activation or dysregulation of the UPR can lead to cell death and other detrimental events such as inflammation and oxidative stress; these processes are implicated in the pathogenesis of many human diseases including retinal disorders. In this review manuscript, we discuss the unique features of the ER and ER stress signaling in the retina and retinal neurons and describe recent advances in the research to uncover the role of ER stress signaling in neurodegenerative retinal diseases including age-related macular degeneration, inherited retinal degeneration, achromatopsia and cone diseases, and diabetic retinopathy. In some chapters, we highlight the complex interactions between the ER and other intracellular organelles focusing on mitochondria and illustrate how ER stress signaling regulates common cellular stress pathways such as autophagy. We also touch upon the integrated stress response in retinal degeneration and diabetic retinopathy. Finally, we provide an update on the current development of pharmacological agents targeting the UPR response and discuss some unresolved questions and knowledge gaps to be addressed by future research.
Keywords: Autophagy; Diabetic retinopathy; Endoplasmic reticulum; Integrated stress response; Mitochondria; Protein homeostasis; Retina; Retinal degeneration; Unfolded protein response.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest None.
Figures




References
-
- Adachi Y, Yamamoto K, Okada T, Yoshida H, Harada A, Mori K, 2008. ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell Struct. Funct 33, 75–89. - PubMed
-
- Almanza A, Mnich K, Blomme A, Robinson CM, Rodriguez-Blanco G, Kierszniowska S, McGrath EP, Le Gallo M, Pilalis E, Swinnen JV, Chatziioannou A, Chevet E, Gorman AM, Samali A, 2022. Regulated IRE1α-dependent decay (RIDD)-mediated reprograming of lipid metabolism in cancer. Nat. Commun 13, 2493. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

