Trends in antifungal resistance in Candida from a multicenter study conducted in Madrid (CANDIMAD study): fluconazole-resistant C. parapsilosis spreading has gained traction in 2022
- PMID: 38092562
- PMCID: PMC10783443
- DOI: 10.1128/aac.00986-23
Trends in antifungal resistance in Candida from a multicenter study conducted in Madrid (CANDIMAD study): fluconazole-resistant C. parapsilosis spreading has gained traction in 2022
Abstract
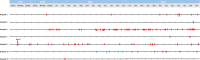
We previously conducted a multicenter surveillance study on Candida epidemiology and antifungal resistance in Madrid (CANDIMAD study; 2019-2021), detecting an increase in fluconazole-resistant Candida parapsilosis. We here present data on isolates collected in 2022. Furthermore, we report the epidemiology and antifungal resistance trends during the entire period, including an analysis per ward of admission. Candida spp. incident isolates from blood cultures and intra-abdominal samples from patients cared for at 16 hospitals in Madrid, Spain, were tested with the EUCAST E.Def 7.3.2 method against amphotericin B, azoles, micafungin, anidulafungin, and ibrexafungerp and were molecularly characterized. In 2022, we collected 766 Candida sp. isolates (686 patients; blood cultures, 48.8%). Candida albicans was the most common species found, and Candida auris was undetected. No resistance to amphotericin B was found. Overall, resistance to echinocandins was low (0.7%), whereas fluconazole resistance was 12.0%, being higher in blood cultures (16.0%) mainly due to fluconazole-resistant C. parapsilosis clones harboring the Y132F-R398I ERG11p substitutions. Ibrexafungerp showed in vitro activity against the isolates tested. Whereas C. albicans was the dominant species in most hospital wards, we observed increasing C. parapsilosis proportions in blood. During the entire period, echinocandin resistance rates remained steadily low, while fluconazole resistance increased in blood from 6.8% (2019) to 16% (2022), mainly due to fluconazole-resistant C. parapsilosis (2.6% in 2019 to 36.6% in 2022). Up to 7 out of 16 hospitals were affected by fluconazole-resistant C. parapsilosis. In conclusion, rampant clonal spreading of C. parapsilosis fluconazole-resistant genotypes is taking place in Madrid.
Keywords: C. parapsilosis; Candida; Madrid; Y132F; antifungal resistance.
Conflict of interest statement
J.G. has received funds for participating in educational activities organized on behalf of Gilead, Pfizer, Mundipharma, and MSD; he has also received research funds from FIS, Gilead, Scynexis, F2G, Mundipharma, and Cidara, outside the submitted work.
Figures



References
-
- Ceballos-Garzon A, Peñuela A, Valderrama-Beltrán S, Vargas-Casanova Y, Ariza B, Parra-Giraldo CM. 2023. Emergence and circulation of azole-resistant C. albicans, C. auris and C. parapsilosis bloodstream isolates carrying Y132F, K143R or T220L Erg11P substitutions in Colombia. Front Cell Infect Microbiol 13:1136217. doi:10.3389/fcimb.2023.1136217 - DOI - PMC - PubMed
-
- Presente S, Bonnal C, Normand AC, Gaudonnet Y, Fekkar A, Timsit JF, Kernéis S. 2023. Hospital clonal outbreak of fluconazole-resistant Candida parapsilosis harboring the Y132F ERG11p substitution in a french intensive care unit. Antimicrob Agents Chemother 67:e0113022. doi:10.1128/aac.01130-22 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

