Spatial Relationships in the Tumor Microenvironment Demonstrate Association with Pathologic Response to Neoadjuvant Chemoimmunotherapy in Muscle-invasive Bladder Cancer
- PMID: 38092611
- PMCID: PMC11022933
- DOI: 10.1016/j.eururo.2023.11.008
Spatial Relationships in the Tumor Microenvironment Demonstrate Association with Pathologic Response to Neoadjuvant Chemoimmunotherapy in Muscle-invasive Bladder Cancer
Abstract
Background: Platinum-based neoadjuvant chemotherapy (NAC) is standard for patients with muscle-invasive bladder cancer (MIBC). Pathologic response (complete: ypT0N0 and partial: <ypT2N0) to NAC is associated with improved survival with ypT0N0 achieved in 30-40% of cases. Strategies to increase response to NAC are needed. Incorporation of immune checkpoint inhibitors (ICIs) has demonstrated promise, and better spatial understanding of the tumor microenvironment may help predict NAC/ICI response.
Objective: Using the NanoString GeoMx platform, we performed proteomic digital spatial profiling (DSP) on transurethral resections of bladder tumors from 18 responders (<ypT2) and 18 nonresponders (≥ypT2) from two studies of NAC (gemcitabine and cisplatin) plus ICI (LCCC1520 [pembrolizumab] and BLASST-1 [nivolumab]).
Design, setting, and participants: Pretreatment tumor samples were stained by hematoxylin and eosin and immunofluorescence (panCK and CD45) to select four regions of interest (ROIs): tumor enriched (TE), immune enriched (IE), tumor/immune interface (tumor interface = TX and immune interface = IX).
Outcome measurements and statistical analysis: DSP was performed with 52 protein markers from immune cell profiling, immunotherapy drug target, immune activation status, immune cell typing, and pan-tumor panels.
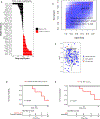
Results and limitations: Protein marker expression patterns were analyzed to determine their association with pathologic response, incorporating or agnostic of their ROI designation (TE/IE/TX/IX). Overall, DSP-based marker expression showed high intratumoral heterogeneity; however, response was associated with markers including PD-L1 (ROI agnostic), Ki-67 (ROI agnostic, TE, IE, and TX), HLA-DR (TX), and HER2 (TE). An elastic net model of response with ROI-inclusive markers demonstrated better validation set performance (area under the curve [AUC] = 0.827) than an ROI-agnostic model (AUC = 0.432). A model including DSP, tumor mutational burden, and clinical data performed no better (AUC = 0.821) than the DSP-only model.
Conclusions: Despite high intratumoral heterogeneity of DSP-based marker expression, we observed associations between pathologic response and specific DSP-based markers in a spatially dependent context. Further exploration of tumor region-specific biomarkers may help predict response to neoadjuvant chemoimmunotherapy in MIBC.
Patient summary: In this study, we used the GeoMx platform to perform proteomic digital spatial profiling on transurethral resections of bladder tumors from 18 responders and 18 nonresponders from two studies of neoadjuvant chemotherapy (gemcitabine and cisplatin) plus immune checkpoint inhibitor therapy (LCCC1520 [pembrolizumab] and BLASST-1 [nivolumab]). We found that assessing protein marker expression in the context of tumor architecture improved response prediction.
Keywords: BLASST-1; Bladder cancer; Digital spatial profiling; Elastic net regression; GeoMx; LCCC1520; Neoadjuvant chemoimmunotherapy.
Copyright © 2023. Published by Elsevier B.V.
Figures




References
-
- International collaboration of Trialists, Medical Research Council Advanced Bladder Cancer Working Party (now the National Cancer Research Institute Bladder Cancer Clinical Studies Group), European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group, et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol 2011;29:2171–7. - PMC - PubMed
-
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859–66. - PubMed
-
- Balar AV, Kamat AM, Kulkarni GS, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol 2021;22:919–30. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

