I n vitro pharmacokinetics/pharmacodynamics of the β-lactamase inhibitor, durlobactam, in combination with sulbactam against Acinetobacter baumannii-calcoaceticus complex
- PMID: 38092676
- PMCID: PMC10869334
- DOI: 10.1128/aac.00312-23
I n vitro pharmacokinetics/pharmacodynamics of the β-lactamase inhibitor, durlobactam, in combination with sulbactam against Acinetobacter baumannii-calcoaceticus complex
Abstract
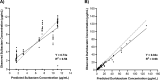
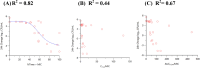
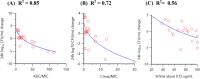
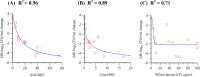
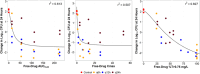
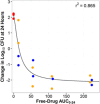
Infections caused by Acinetobacter baumannii are increasingly multidrug resistant and associated with high rates of morbidity and mortality. Sulbactam is a β-lactamase inhibitor with intrinsic antibacterial activity against A. baumannii. Durlobactam is a non-β-lactam β-lactamase inhibitor with an extended spectrum of activity compared to other inhibitors of its class. In vitro pharmacodynamic infection models were undertaken to establish the pharmacokinetic/pharmacodynamic (PK/PD) index and magnitudes associated with sulbactam and durlobactam efficacy and to simulate epithelial lining fluid (ELF) exposures at clinical doses to understand sulbactam-durlobactam activity with and without co-administration of a carbapenem. Hollow fiber infection models (HFIMs) and one-compartment systems were used to identify the PK/PD indices and exposure magnitudes associated of 1-log10 and 2-log10 colony-forming unit (CFU)/mL reductions. Sulbactam and durlobactam demonstrated PK/PD drivers of % time above the minimum inhibition concentration (%T > MIC) and area under the plasma concentration-time curve from time 0 to 24 h (AUC0-24)/MIC, respectively. Against a sulbactam-susceptible strain, sulbactam %T > MIC of 71.5 and 82.0 were associated with 1-log10 and 2-log10 CFU/mL reductions, respectively, in the HFIM. Against a non-susceptible strain, durlobactam restored the activity of sulbactam with an AUC0-24/MICs of 34.0 and 46.8 using a polysulfone cartridge to achieve a 1-log10 and 2-log10 CFU/mL reduction. These magnitudes were reduced to 13.8 and 24.2, respectively, using a polyvinylidene fluoride cartridge with a membrane pore size of 0.1 μm. In the one-compartment model, durlobactam AUC0-24/MIC to achieve 1-log10 and 2-log10 CFU/mL reduction were 7.6 and 33.4, respectively. Simulations of clinical ELF exposures in the HFIM showed cidal activity at MICs ≤4 µg/mL. Penicillin binding protein 3 mutant strains with MICs of 8 μg/mL may benefit from the addition of a carbapenem at clinical exposures.
Keywords: Acinetobacter calcoaceticus; durlobactam; pharmacodynamics; pharmacokinetics; sulbactam.
Conflict of interest statement
J.O., A.T., A.C., and S.M.M. are paid employees of Entasis Therapeutics, a wholly owned subsidiary of Innoviva, Inc.
Figures



References
-
- Cai B, Echols R, Magee G, Arjona Ferreira JC, Morgan G, Ariyasu M, Sawada T, Nagata TD. 2017. Prevalence of carbapenem-resistant Gram-negative infections in the United States predominated by Acinetobacter baumannii and Pseudomonas aeruginosa. Open Forum Infect Dis 4:fx176. doi:10.1093/ofid/ofx176 - DOI - PMC - PubMed
-
- Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. 2022. Infectious diseases society of America guidance on the treatment of AmpC β-lactamase-producing enterobacterales, carbapenem-resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia infections. Clin Infect Dis 74:2089–2114. doi:10.1093/cid/ciab1013 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

