Niacin restriction with NAMPT-inhibition is synthetic lethal to neuroendocrine carcinoma
- PMID: 38092728
- PMCID: PMC10719245
- DOI: 10.1038/s41467-023-43630-3
Niacin restriction with NAMPT-inhibition is synthetic lethal to neuroendocrine carcinoma
Abstract
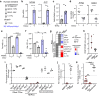
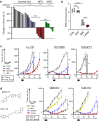
Nicotinamide phosphoribosyltransferase (NAMPT) plays a major role in NAD biosynthesis in many cancers and is an attractive potential cancer target. However, factors dictating therapeutic efficacy of NAMPT inhibitors (NAMPTi) are unclear. We report that neuroendocrine phenotypes predict lung and prostate carcinoma vulnerability to NAMPTi, and that NAMPTi therapy against those cancers is enhanced by dietary modification. Neuroendocrine differentiation of tumor cells is associated with down-regulation of genes relevant to quinolinate phosphoribosyltransferase-dependent de novo NAD synthesis, promoting NAMPTi susceptibility in vitro. We also report that circulating nicotinic acid riboside (NAR), a non-canonical niacin absent in culture media, antagonizes NAMPTi efficacy as it fuels NAMPT-independent but nicotinamide riboside kinase 1-dependent NAD synthesis in tumors. In mouse transplantation models, depleting blood NAR by nutritional or genetic manipulations is synthetic lethal to tumors when combined with NAMPTi. Our findings provide a rationale for simultaneous targeting of NAR metabolism and NAMPT therapeutically in neuroendocrine carcinoma.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
- 21K19420/MEXT | Japan Society for the Promotion of Science (JSPS)
- 19H01036/MEXT | Japan Society for the Promotion of Science (JSPS)
- 20K17203/MEXT | Japan Society for the Promotion of Science (JSPS)
- 22K08989/MEXT | Japan Society for the Promotion of Science (JSPS)
- 19K09469/MEXT | Japan Society for the Promotion of Science (JSPS)
- 21K19748/MEXT | Japan Society for the Promotion of Science (JSPS)
- 21K16531/MEXT | Japan Society for the Promotion of Science (JSPS)
- 21K09482/MEXT | Japan Society for the Promotion of Science (JSPS)
- 23ama221122h0001/Japan Agency for Medical Research and Development (AMED)
- NA/Princess Takamatsu Cancer Research Fund
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

