Chlorpromazine affects glioblastoma bioenergetics by interfering with pyruvate kinase M2
- PMID: 38092755
- PMCID: PMC10719363
- DOI: 10.1038/s41419-023-06353-3
Chlorpromazine affects glioblastoma bioenergetics by interfering with pyruvate kinase M2
Abstract
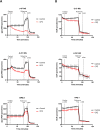
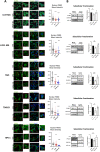
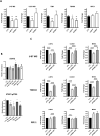
Glioblastoma (GBM) is the most frequent and lethal brain tumor, whose therapeutic outcome - only partially effective with current schemes - places this disease among the unmet medical needs, and effective therapeutic approaches are urgently required. In our attempts to identify repositionable drugs in glioblastoma therapy, we identified the neuroleptic drug chlorpromazine (CPZ) as a very promising compound. Here we aimed to further unveil the mode of action of this drug. We performed a supervised recognition of the signal transduction pathways potentially influenced by CPZ via Reverse-Phase Protein microArrays (RPPA) and carried out an Activity-Based Protein Profiling (ABPP) followed by Mass Spectrometry (MS) analysis to possibly identify cellular factors targeted by the drug. Indeed, the glycolytic enzyme PKM2 was identified as one of the major targets of CPZ. Furthermore, using the Seahorse platform, we analyzed the bioenergetics changes induced by the drug. Consistent with the ability of CPZ to target PKM2, we detected relevant changes in GBM energy metabolism, possibly attributable to the drug's ability to inhibit the oncogenic properties of PKM2. RPE-1 non-cancer neuroepithelial cells appeared less responsive to the drug. PKM2 silencing reduced the effects of CPZ. 3D modeling showed that CPZ interacts with PKM2 tetramer in the same region involved in binding other known activators. The effect of CPZ can be epitomized as an inhibition of the Warburg effect and thus malignancy in GBM cells, while sparing RPE-1 cells. These preclinical data enforce the rationale that allowed us to investigate the role of CPZ in GBM treatment in a recent multicenter Phase II clinical trial.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



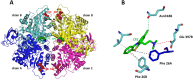
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

