Cortical reactivations predict future sensory responses
- PMID: 38093002
- PMCID: PMC11014741
- DOI: 10.1038/s41586-023-06810-1
Cortical reactivations predict future sensory responses
Abstract
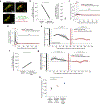
Many theories of offline memory consolidation posit that the pattern of neurons activated during a salient sensory experience will be faithfully reactivated, thereby stabilizing the pattern1,2. However, sensory-evoked patterns are not stable but, instead, drift across repeated experiences3-6. Here, to investigate the relationship between reactivations and the drift of sensory representations, we imaged the calcium activity of thousands of excitatory neurons in the mouse lateral visual cortex. During the minute after a visual stimulus, we observed transient, stimulus-specific reactivations, often coupled with hippocampal sharp-wave ripples. Stimulus-specific reactivations were abolished by local cortical silencing during the preceding stimulus. Reactivations early in a session systematically differed from the pattern evoked by the previous stimulus-they were more similar to future stimulus response patterns, thereby predicting both within-day and across-day representational drift. In particular, neurons that participated proportionally more or less in early stimulus reactivations than in stimulus response patterns gradually increased or decreased their future stimulus responses, respectively. Indeed, we could accurately predict future changes in stimulus responses and the separation of responses to distinct stimuli using only the rate and content of reactivations. Thus, reactivations may contribute to a gradual drift and separation in sensory cortical response patterns, thereby enhancing sensory discrimination7.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures


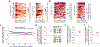


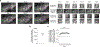







References
-
- Foster DJ Replay comes of age. Annu. Rev. Neurosci 40, 581–602 (2017). - PubMed
-
- Failor SW, Carandini M & Harris KD Visuomotor association orthogonalizes visual cortical population codes. Preprint at bioRxiv 10.1101/2021.05.23.445338 (2022). - DOI
-
- Schoonover CE et al. Representational drift in primary olfactory cortex. Nature 594, 541–546 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

