Real-world clinical outcomes and treatment patterns in patients with MDD treated with vortioxetine: a retrospective study
- PMID: 38093196
- PMCID: PMC10720213
- DOI: 10.1186/s12888-023-05439-8
Real-world clinical outcomes and treatment patterns in patients with MDD treated with vortioxetine: a retrospective study
Abstract
Background: This study included evaluation of the effectiveness of vortioxetine, a treatment for adults with major depressive disorder (MDD), using patient-reported outcome measures (PROMs) in a real-world setting.
Methods: This retrospective chart review analyzed the care experiences of adult patients with a diagnosis of MDD from Parkview Physicians Group - Mind-Body Medicine, Midwestern United States. Patients with a prescription for vortioxetine, an initial baseline visit, and ≥ 2 follow-up visits within 16 weeks from September 2014 to December 2018 were included. The primary outcome measure was effectiveness of vortioxetine on depression severity as assessed by change in Patient Health Questionnaire-9 (PHQ-9) scores ~ 12 weeks after initiation of vortioxetine. Secondary outcomes included changes in depression-related symptoms (i.e., sexual dysfunction, sleep disturbance, cognitive function, work/social function), clinical characteristics, response, remission, and medication persistence. Clinical narrative notes were also analyzed to examine sleep disturbance, sexual dysfunction, appetite, absenteeism, and presenteeism. All outcomes were examined at index (start of vortioxetine) and at ~ 12 weeks, and mean differences were analyzed using pairwise t tests.
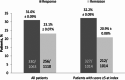
Results: A total of 1242 patients with MDD met inclusion criteria, and 63.9% of these patients had ≥ 3 psychiatric diagnoses and 65.9% were taking ≥ 3 medications. PHQ-9 mean scores decreased significantly from baseline to week 12 (14.15 ± 5.8 to 9.62 ± 6.03, respectively; p < 0.001). At week 12, the response and remission rates in all patients were 31.0% and 23.1%, respectively, and 67% continued vortioxetine treatment. Overall, results also showed significant improvements by week 12 in anxiety (p < 0.001), sexual dysfunction (p < 0.01), sleep disturbance (p < 0.01), cognitive function (p < 0.001), work/social functioning (p = 0.021), and appetite (p < 0.001). A significant decrease in presenteeism was observed at week 12 (p < 0.001); however, no significant change was observed in absenteeism (p = 0.466).
Conclusions: Using PROMs, our study results suggest that adults with MDD prescribed vortioxetine showed improvement in depressive symptoms in the context of a real-world clinical practice setting. These patients had multiple comorbid psychiatric and physical diagnoses and multiple previous antidepressant treatments had failed.
Keywords: Cognitive functioning; Comorbid psychiatric diagnoses; Depression severity; Major depressive disorder; Patient-reported outcome measures; Previous antidepressant treatments; Real-world clinical practice setting; Sexual dysfunction; Social functioning; Vortioxetine.
© 2023. The Author(s).
Conflict of interest statement
Brandon T. McDaniel, Victor Cornet, and Jeanne Carroll are employees of Parkview Mirro Center for Research and Innovation, which received funding from Takeda for the study. Victor Cornet also holds stock in Axcella Health. Joseph Chudzik is an intern/contractor with Parkview Mirro Center. Lambros Chrones, Maggie McCue, Sara Sarkey, and Betty Lorenz are employees of Takeda Pharmaceuticals U.S.A., Inc. Lambros Chrones, Maggie McCue, and Sara Sarkey have stock or stock options with Takeda. Betty Lorenz received funding for attending meetings and/or travel from Takeda and holds stock options with Takeda. Debra F. Lawrence was an employee of Takeda Pharmaceuticals U.S.A., Inc., at the time of the study and holds stock or stock options with Takeda. Shion Guha is an external contractor for Parkview Mirro Center, which received funds from Takeda for the study, and is an employee of the University of Toronto. Jay Fawver was a consultant with Takeda Pharmaceuticals U.S.A., Inc., at the time of the study and is an employee of Parkview Physicians Group – Mind-Body Medicine. He also is on the speakers’ bureau for Takeda. Jeanette Cochran is an employee of Parkview Physicians Group – Mind-Body Medicine.
Figures
Similar articles
-
Effectiveness of vortioxetine in patients with major depressive disorder and co-morbid generalized anxiety disorder in routine clinical practice: A subgroup analysis of the RELIEVE study.J Psychopharmacol. 2023 Mar;37(3):279-288. doi: 10.1177/02698811221132468. Epub 2022 Nov 15. J Psychopharmacol. 2023. PMID: 36377523 Free PMC article. Clinical Trial.
-
Long-term functioning outcomes are predicted by cognitive symptoms in working patients with major depressive disorder treated with vortioxetine: results from the AtWoRC study.CNS Spectr. 2019 Dec;24(6):616-627. doi: 10.1017/S1092852919000786. CNS Spectr. 2019. PMID: 30802419 Clinical Trial.
-
Safety and effectiveness of vortioxetine in patients with major depressive disorder in a real-life clinical setting in India: results from an interventional, flexible-dose study.Curr Med Res Opin. 2024 Sep;40(9):1637-1645. doi: 10.1080/03007995.2024.2382773. Epub 2024 Aug 7. Curr Med Res Opin. 2024. PMID: 39110846 Clinical Trial.
-
Novel Augmentation Strategies in Major Depression.Dan Med J. 2017 Apr;64(4):B5338. Dan Med J. 2017. PMID: 28385173 Review.
-
Effectiveness and Safety of Vortioxetine for the Treatment of Major Depressive Disorder in the Real World: A Systematic Review and Meta-Analysis.Int J Neuropsychopharmacol. 2023 Jun 23;26(6):373-384. doi: 10.1093/ijnp/pyad018. Int J Neuropsychopharmacol. 2023. PMID: 37105713 Free PMC article.
Cited by
-
Switching to Vortioxetine in Patients with Poorly Tolerated Antidepressant-Related Sexual Dysfunction in Clinical Practice: A 3-Month Prospective Real-Life Study.J Clin Med. 2024 Jan 18;13(2):546. doi: 10.3390/jcm13020546. J Clin Med. 2024. PMID: 38256680 Free PMC article.
-
Mechanisms Involved in the Link between Depression, Antidepressant Treatment, and Associated Weight Change.Int J Mol Sci. 2024 Apr 20;25(8):4511. doi: 10.3390/ijms25084511. Int J Mol Sci. 2024. PMID: 38674096 Free PMC article. Review.
-
Post-marketing safety surveillance of vortioxetine hydrobromide: a pharmacovigilance study leveraging FAERS database.Front Psychiatry. 2025 Feb 24;16:1532803. doi: 10.3389/fpsyt.2025.1532803. eCollection 2025. Front Psychiatry. 2025. PMID: 40066137 Free PMC article.
References
-
- Czeisler ME, Lane RI, Petrosky E, Wiley JF, Christensen A, Njai R, Weaver MD, Robbins R, Facer-Childs ER, Barger LK, Czeisler CA, et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic—United States. MMWR Morb Mortal Wkly Rep. 2020;69:24–30. doi: 10.15585/mmwr.mm6932a1. - DOI - PMC - PubMed
-
- IsHak WW, Mirocha J, James D, Tobia G, Vilhauer J, Fakhry H, Pi S, Hanson E, Nashawati R, Peselow ED, et al. Quality of life in major depressive disorder before/after multiple steps of treatment and one-year follow-up. Acta Psychiatr Scand. 2015;131:51–60. doi: 10.1111/acps.12301. - DOI - PMC - PubMed
-
- Papakostas GI, Jackson WC, Rafeyan R, Trivedi MH. Inadequate response to antidepressant treatment in major depressive disorder. J Clin Psychiatry. 2020;81(30). 10.4088/JCP.OT19037COM5. OT19037COM5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

