Nutlin-3a induces KRAS mutant/p53 wild type lung cancer specific methuosis-like cell death that is dependent on GFPT2
- PMID: 38093368
- PMCID: PMC10720203
- DOI: 10.1186/s13046-023-02922-8
Nutlin-3a induces KRAS mutant/p53 wild type lung cancer specific methuosis-like cell death that is dependent on GFPT2
Abstract
Background: Oncogenic KRAS mutation, the most frequent mutation in non-small cell lung cancer (NSCLC), is an aggressiveness risk factor and leads to the metabolic reprogramming of cancer cells by promoting glucose, glutamine, and fatty acid absorption and glycolysis. Lately, sotorasib was approved by the FDA as a first-in-class KRAS-G12C inhibitor. However, sotorasib still has a derivative barrier, which is not effective for other KRAS mutation types, except for G12C. Additionally, resistance to sotorasib is likely to develop, demanding the need for alternative therapeutic strategies.
Methods: KRAS mutant, and wildtype NSCLC cells were used in vitro cell analyses. Cell viability, proliferation, and death were measured by MTT, cell counting, colony analyses, and annexin V staining for FACS. Cell tracker dyes were used to investigate cell morphology, which was examined by holotomograpy, and confocal microscopes. RNA sequencing was performed to identify key target molecule or pathway, which was confirmed by qRT-PCR, western blotting, and metabolite analyses by UHPLC-MS/MS. Zebrafish and mouse xenograft model were used for in vivo analysis.
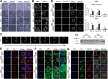
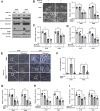
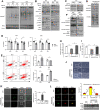
Results: In this study, we found that nutlin-3a, an MDM2 antagonist, inhibited the KRAS-PI3K/Akt-mTOR pathway and disrupted the fusion of both autophagosomes and macropinosomes with lysosomes. This further elucidated non-apoptotic and catastrophic macropinocytosis associated methuosis-like cell death, which was found to be dependent on GFPT2 of the hexosamine biosynthetic pathway, specifically in KRAS mutant /p53 wild type NSCLC cells.
Conclusion: These results indicate the potential of nutlin-3a as an alternative agent for treating KRAS mutant/p53 wild type NSCLC cells.
Keywords: GFPT2; HBP; KRAS mutation; Macropinocytosis; Methuosis; Nutlin-3a.
© 2023. The Author(s).
Conflict of interest statement
No.
Figures





References
-
- FDA Approves First KRAS Inhibitor: Sotorasib. Cancer Discov. 2021;11(8):Of4. 10.1158/2159-8290.CD-NB2021-0362. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

