Development of potent and effective SARS-CoV-2 main protease inhibitors based on maleimide analogs for the potential treatment of COVID-19
- PMID: 38093611
- PMCID: PMC10732195
- DOI: 10.1080/14756366.2023.2290910
Development of potent and effective SARS-CoV-2 main protease inhibitors based on maleimide analogs for the potential treatment of COVID-19
Abstract
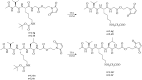
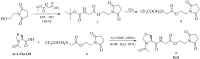
In the present work, we report a new series of potent SARS-CoV-2 Main Protease (Mpro) inhibitors based on maleimide derivatives. The inhibitory activities were tested in an enzymatic assay using recombinant Mpro (3CL Protease from coronavirus SARS-CoV-2). Within the set of new Mpro inhibitors, 6e demonstrated the highest activity in the enzymatic assay with an IC50 value of 8.52 ± 0.44 µM. The IC50 value for Nirmatrelvir (PF-07321332, used as a reference) was 0.84 ± 0.37 µM. The cytotoxic properties were determined in the MTT assay using MRC-5 and HEK-293 cell lines. In the course of the investigation, we found that the newly obtained maleimide derivatives are not substantially cytotoxic (IC50 values for most compounds were above 200 µM).
Keywords: COVID-19; Coronavirus SARS-CoV-2; SARS-CoV-2 main protease mpro inhibitors; maleimide derivatives.
Conflict of interest statement
The authors report no conflict of interest.
Figures


Similar articles
-
Glycyrrhizic acid conjugates with amino acid methyl esters target the main protease, exhibiting antiviral activity against wild-type and nirmatrelvir-resistant SARS-CoV-2 variants.Antiviral Res. 2024 Jul;227:105920. doi: 10.1016/j.antiviral.2024.105920. Epub 2024 May 29. Antiviral Res. 2024. PMID: 38821317
-
Exploration of P1 and P4 modifications of nirmatrelvir: Design, synthesis, biological evaluation, and X-ray structural studies of SARS-CoV-2 Mpro inhibitors.Eur J Med Chem. 2024 Mar 5;267:116132. doi: 10.1016/j.ejmech.2024.116132. Epub 2024 Feb 1. Eur J Med Chem. 2024. PMID: 38335815 Free PMC article.
-
In silico investigation of Komaroviquinone as a potential inhibitor of SARS-CoV-2 main protease (Mpro): Molecular docking, molecular dynamics, and QM/MM approaches.J Mol Graph Model. 2024 Jan;126:108662. doi: 10.1016/j.jmgm.2023.108662. Epub 2023 Nov 7. J Mol Graph Model. 2024. PMID: 37950976
-
Substitutions in SARS-CoV-2 Mpro Selected by Protease Inhibitor Boceprevir Confer Resistance to Nirmatrelvir.Viruses. 2023 Sep 21;15(9):1970. doi: 10.3390/v15091970. Viruses. 2023. PMID: 37766376 Free PMC article. Review.
-
[Nirmatrelvir plus ritonavir (Paxlovid) a potent SARS-CoV-2 3CLpro protease inhibitor combination].Rev Esp Quimioter. 2022 Jun;35(3):236-240. doi: 10.37201/req/002.2022. Epub 2022 Feb 21. Rev Esp Quimioter. 2022. PMID: 35183067 Free PMC article. Review. Spanish.
Cited by
-
Design, Synthesis, and Antitumor Evaluation of an Opioid Growth Factor Bioconjugate Targeting Pancreatic Ductal Adenocarcinoma.Pharmaceutics. 2024 Feb 16;16(2):283. doi: 10.3390/pharmaceutics16020283. Pharmaceutics. 2024. PMID: 38399336 Free PMC article.
-
The Dynamical Asymmetry in SARS-CoV2 Protease Reveals the Exchange Between Catalytic Activity and Stability in Homodimers.Molecules. 2025 Mar 22;30(7):1412. doi: 10.3390/molecules30071412. Molecules. 2025. PMID: 40286026 Free PMC article.
References
-
- Anand K, Ziebuhr J, Wadhwani P, Mesters JR, Hilgenfeld R.. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. (5626)2003; 300:1763–1767. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
