A comparison of treatment response to biologics in asthma-COPD overlap and pure asthma: Findings from the PRISM study
- PMID: 38093952
- PMCID: PMC10716772
- DOI: 10.1016/j.waojou.2023.100848
A comparison of treatment response to biologics in asthma-COPD overlap and pure asthma: Findings from the PRISM study
Abstract
Background: Despite the increasing use of biologics in severe asthma, there is limited research on their use in asthma-chronic obstructive pulmonary disease overlap (ACO). We compared real-world treatment responses to biologics in ACO and asthma.
Methods: We conducted a multicenter, retrospective, cohort study using data from the Precision Medicine Intervention in Severe Asthma (PRISM). ACO was defined as post-bronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) <0.7 and a smoking history of >10 pack-years. Physicians selected biologics (omalizumab, mepolizumab, reslizumab, benralizumab, and dupilumab) based on each United States Food & Drug Administration (FDA) approval criteria.
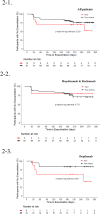
Results: After six-month treatment with biologics, both patients with ACO (N = 13) and asthma (N = 81) showed positive responses in FEV1 (10.69 ± 17.17 vs. 11.25 ± 12.87 %, P = 0.652), Asthma Control Test score (3.33 ± 5.47 vs. 5.39 ± 5.42, P = 0.290), oral corticosteroid use (-117.50 ± 94.38 vs. -115.06 ± 456.85 mg, P = 0.688), fractional exhaled nitric oxide levels (-18.62 ± 24.68 vs. -14.66 ± 45.35 ppb, P = 0.415), sputum eosinophils (-3.40 ± 10.60 vs. -14.48 ± 24.01 %, P = 0.065), blood eosinophils (-36.47 ± 517.02 vs. -363.22 ± 1294.59, P = 0.013), and exacerbation frequency (-3.07 ± 4.42 vs. -3.19 ± 5.11, P = 0.943). The odds ratio for exacerbation and time-to-first exacerbation showed no significant difference after full adjustments, and subgroup analysis according to biologic type was also showed similar results.
Conclusions: Biologics treatment response patterns in patients with ACO and asthma were comparable, suggesting that biologics should be actively considered for ACO patients as well.
Keywords: Asthma; Asthma-COPD overlap; Biologics; Monoclonal antibodies; Treatment response.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Barczyk A., Maskey-Warzęchowska M., Górska K., et al. Asthma-COPD overlap-A discordance between patient populations defined by different diagnostic criteria. J Allergy Clin Immunol Pract. 2019;7(7):2326–2336.e2325. - PubMed
-
- Leung C., Sin D.D. Asthma-COPD overlap: what are the important questions? Chest. 2022;161(2):330–344. - PubMed
-
- Global Strategy for Asthma Management and Prevention . 2014. Global Initiative for Asthma.
LinkOut - more resources
Full Text Sources

