Repurposing of Rutan showed effective treatment for COVID-19 disease
- PMID: 38093975
- PMCID: PMC10716264
- DOI: 10.3389/fmed.2023.1310129
Repurposing of Rutan showed effective treatment for COVID-19 disease
Abstract
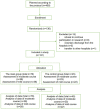
Previously, from the tannic sumac plant (Rhus coriaria), we developed the Rutan 25 mg oral drug tablets with antiviral activity against influenza A and B viruses, adenoviruses, paramyxoviruses, herpes virus, and cytomegalovirus. Here, our re-purposing study demonstrated that Rutan at 25, 50, and 100 mg/kg provided a very effective and safe treatment for COVID-19 infection, simultaneously inhibiting two vital enzyme systems of the SARS-CoV-2 virus: 3C-like proteinase (3CLpro) and RNA-dependent RNA polymerase (RdRp). There was no drug accumulation in experimental animals' organs and tissues. A clinical study demonstrated a statistically significant decrease in the C-reactive protein and a reduction of the viremia period. In patients receiving Rutan 25 mg (children) and 100 mg (adults), the frequency of post-COVID-19 manifestations was significantly less than in the control groups not treated with Rutan tablets. Rutan, having antiviral activity, can provide safe treatment and prevention of COVID-19 in adults and children.
Clinical trial registration: ClinicalTrials.gov, ID NCT05862883.
Keywords: 3CLpro; COVID-19; RdRp; Rhus coriaria; Rutan; SARS-CoV-2; clinical trial.
Copyright © 2023 Salikhov, Abdurakhmonov, Oshchepkova, Ziyavitdinov, Berdiev, Aisa, Shen, Xu, Xu, Jiang, Zhang, Vypova, Allaberganov, Tagayalieva, Musabaev, Ibadova, Rajabov and Lokteva.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

