Dithione, the antipodal redox partner of ene-1,2-dithiol ligands and their metal complexes
- PMID: 38094102
- PMCID: PMC10718511
- DOI: 10.1016/j.ccr.2020.213211
Dithione, the antipodal redox partner of ene-1,2-dithiol ligands and their metal complexes
Abstract
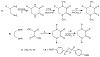
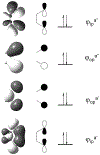
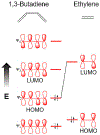
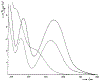
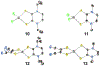
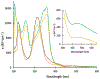
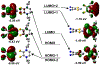
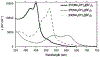
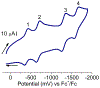
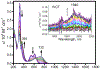
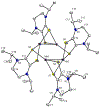
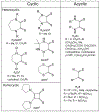
Defining the oxidation state of the central atom in a coordination compound is fundamental in understanding the electronic structure and provides a starting point for elucidating molecular properties. The presence of non-innocent ligand(s) can obscure the oxidation state of the central atom as the ligand contribution to the electronic structure is difficult to ascertain. Redox-active ligands, such as dithiolene ligands, are well known non-innocent ligands that can exist in both a fully reduced (Dt2-) and fully oxidized (Dt0) states. Complexes containing the fully oxidized dithione state of the ligand are uncommon and only a few have been completely characterized. Dithione ligands are of interest due to their electron-deficient nature and ability to act as an electron acceptor for more electron-rich moieties, such as other dithiolene ligands or metal centers. This article focuses the syntheses, structures, and metal coordination, particularly coordination compounds, of dithione ligands. Various examples of mono, bis, and tris dithione complexes are discussed.
Keywords: Coordination compound; Diels-Alder reaction; Dithiolene; Dithione; Donor-acceptor system; Electron-deficient ligand.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
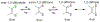
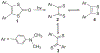

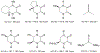
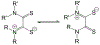








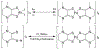





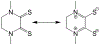

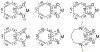

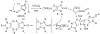

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
