A novel transcranial photobiomodulation device to address motor signs of Parkinson's disease: a parallel randomised feasibility study
- PMID: 38094162
- PMCID: PMC10716000
- DOI: 10.1016/j.eclinm.2023.102338
A novel transcranial photobiomodulation device to address motor signs of Parkinson's disease: a parallel randomised feasibility study
Abstract
Background: Parkinson's disease is a progressive neurological disease with limited treatment options. Animal models and a proof-of-concept case series have suggested that photobiomodulation may be an effective adjunct treatment for the symptoms of Parkinson's disease. The aim was to determine the safety and feasibility of transcranial photobiomodulation (tPBM) to reduce the motor signs of Parkinson's disease.
Methods: In this double-blind, randomised, sham-controlled feasibility trial, patients (aged 59-85 years) with idiopathic Parkinson's disease were treated with a tPBM helmet for 12 weeks (72 treatments with either active or sham therapy; stage 1). Treatment was delivered in the participants' homes, monitored by internet video conferencing (Zoom). Stage 1 was followed by 12 weeks of no treatment for those on active therapy (active-to-no-treatment group), and 12 weeks of active treatment for those on sham (sham-to-active group), for participants who chose to continue (stage 2). The active helmet device delivered red and infrared light to the head for 24 min, 6 days per week. The primary endpoints were safety and motor signs, as assessed by a modified Movement Disorders Society revision of the Unified Parkinson's Disease Rating Scale Part III (MDS-UPDRS-III)-motor scale. This trial is registered with ANZCTR, ACTRN 12621001722886.
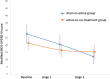
Findings: Between Dec 6, 2021, and Aug 12, 2022, 20 participants were randomly allocated to each of the two groups (10 females plus 10 males per group). All participants in the active group and 18 in the sham group completed 12 weeks of treatment. 14 participants in the sham group chose to continue to active treatment and 12 completed the full 12 weeks of active treatment. Treatment was well tolerated and feasible to deliver, with only minor, temporary adverse events. Of the nine suspected adverse events that were identified, two minor reactions may have been attributable to the device in the sham-to-active group during the active treatment weeks of the trial. One participant experienced temporary leg weakness. A second participant reported decreased fine motor function in the right hand. Both participants continued the trial. The mean modified MDS-UPDRS-III scores for the sham-to-active group at baseline, after 12 weeks of sham treatment, and after 12 weeks of active treatment were 26.8 (sd 14.6), 20.4 (sd 12.8), and 12.2 (sd 8.9), respectively, and for the active-to-no-treatment group these values were 21.3 (sd 9.4), 16.5 (sd 9.4), and 15.3 (sd 10.8), respectively. There was no significant difference between groups at any assessment point. The mean difference between groups at baseline was 5.5 (95% confidence interval (CI) -2.4 to 13.4), after stage 1 was 3.9 (95% CI -3.5 to 11.3 and after stage 2 was -3.1 (95% CI 2.7 to -10.6).
Interpretation: Our findings add to the evidence base to suggest that tPBM is a safe, tolerable, and feasible non-pharmaceutical adjunct therapy for Parkinson's disease. While future work is needed our results lay the foundations for an adequately powered randomised placebo-controlled clinical trial.
Funding: SYMBYX Pty Ltd.
Keywords: Movement disorders; Parkinson's disease; Photobiomodulation; Transcranial; UPDRS.
© 2023 The Author(s).
Conflict of interest statement
AL, BB, CSM and HK are shareholders of SYMBYX Pty Ltd; All other authors declare no competing interests.
Figures

References
-
- McGregor M.M., Nelson A.B. Circuit mechanisms of Parkinson's disease. Neuron. 2019;101(6):1042–1056. - PubMed
-
- Chow R.T., Johnson M.I., Lopes-Martins R.A., Bjordal J.M. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet. 2009;374(9705):1897–1908. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

