Artificial intelligence to improve ischemia prediction in Rubidium Positron Emission Tomography-a validation study
- PMID: 38094578
- PMCID: PMC10713509
- DOI: 10.1007/s13167-023-00341-5
Artificial intelligence to improve ischemia prediction in Rubidium Positron Emission Tomography-a validation study
Abstract
Background: Patients are referred to functional coronary artery disease (CAD) testing based on their pre-test probability (PTP) to search for myocardial ischemia. The recommended prediction tools incorporate three variables (symptoms, age, sex) and are easy to use, but have a limited diagnostic accuracy. Hence, a substantial proportion of non-invasive functional tests reveal no myocardial ischemia, leading to unnecessary radiation exposure and costs. Therefore, preselection of patients before ischemia testing needs to be improved using a more predictive and personalised approach.
Aims: Using multiple variables (symptoms, vitals, ECG, biomarkers), artificial intelligence-based tools can provide a detailed and individualised profile of each patient. This could improve PTP assessment and provide a more personalised diagnostic approach in the framework of predictive, preventive and personalised medicine (PPPM).
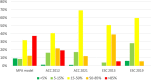
Methods: Consecutive patients (n = 2417) referred for Rubidium-82 positron emission tomography were evaluated. PTP was calculated using the ESC 2013/2019 and ACC 2012/2021 guidelines, and a memetic pattern-based algorithm (MPA) was applied incorporating symptoms, vitals, ECG and biomarkers. Five PTP categories from very low to very high PTP were defined (i.e., < 5%, 5-15%, 15-50%, 50-85%, > 85%). Ischemia was defined as summed difference score (SDS) ≥ 2.
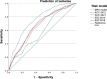
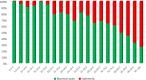
Results: Ischemia was present in 37.1%. The MPA model was most accurate to predict ischemia (AUC: 0.758, p < 0.001 compared to ESC 2013, 0.661; ESC 2019, 0.673; ACC 2012, 0.585; ACC 2021, 0.667). Using the < 5% threshold, the MPA's sensitivity and negative predictive value to rule out ischemia were 99.1% and 96.4%, respectively. The model allocated patients more evenly across PTP categories, reduced the proportion of patients in the intermediate (15-85%) range by 29% (ACC 2012)-51% (ESC 2019), and was the only tool to correctly predict ischemia prevalence in the very low PTP category.
Conclusion: The MPA model enhanced ischemia testing according to the PPPM framework:The MPA model improved individual prediction of ischemia significantly and could safely exclude ischemia based on readily available variables without advanced testing ("predictive").It reduced the proportion of patients in the intermediate PTP range. Therefore, it could be used as a gatekeeper to prevent patients from further unnecessary downstream testing, radiation exposure and costs ("preventive").Consequently, the MPA model could transform ischemia testing towards a more personalised diagnostic algorithm ("personalised").
Supplementary information: The online version contains supplementary material available at 10.1007/s13167-023-00341-5.
Keywords: Artificial intelligence; Coronary artery disease (CAD); Gatekeeper; Improved individual outcome; Ischemia; Patient stratification; Positron emission tomography (PET); Predictive preventive personalised medicine (PPPM/3PM); Pretest probability (PTP); Risk stratification.
© The Author(s) 2023.
Conflict of interest statement
Competing interestsSimon M. Frey: nothing to declare. Adam Bakula: nothing to declare. Andrew Tsirkin: head modeling and development of Exploris Health. Vasily Vasilchenko: developer of MPA model at Exploris Health. Peter Ruff: CEO of Exploris Health, stock owner Exploris Health. Caroline Oehri: chief operating officer at Exploris Health, stock owner Exploris Health. Melissa F. Amrein: nothing to declare. Gabrielle Huré: nothing to declare. Klara Rumora: nothing to declare. Ibrahim Schäfer: nothing to declare. Federico Caobelli: nothing to declare. Philip Haaf: nothing to declare. Christian E. Mueller: no conflict of interest to declare regarding this project. Dr. Mueller has received research support from the Swiss National Science Foundation, the Swiss Heart Foundation, the KTI, the University Hospital Basel, the University of Basel, Abbott, Astra Zeneca, Beckman Coulter, Idorsia, Novartis, Ortho Diagnostics, Quidel, Roche, Siemens, Singulex, SpinChip and Sphingotec, as well as speaker honoraria/consulting honoraria from Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, BMS, Idorsia, Novartis, Osler, Roche, Sanofi and SpinChip. Bjoern Andrew Remppis: advisory board member Exploris Health. Hans-Peter Brunner-La Rocca: advisory board member Exploris Health, stock owner Exploris Health, unrestricted research grant by and advisor to Roche Diagnostics. Michael J. Zellweger: advisory board member Exploris Health, stock owner Exploris Health.
Figures
References
-
- World Health O. Mortality and global health estimates. https://www.who.int/data/gho/data/themes/mortality-and-global-health-est.... Accessed 13 Dec 2022.
-
- Jouni H, Askew JW, Crusan DJ, Miller TD, Gibbons RJ. Temporal trends of single-photon emission computed tomography myocardial perfusion imaging in patients with coronary artery disease. Circ Cardiovasc Imaging 2017;10(7) 10.1161/CIRCIMAGING.116.005628. - PubMed
-
- Lertsburapa K, Ahlberg AW, Bateman TM, et al. Independent and incremental prognostic value of left ventricular ejection fraction determined by stress gated rubidium 82 PET imaging in patients with known or suspected coronary artery disease. J Nucl Cardiol. 2008;15(6):745–53. 10.1007/BF03007355. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

