Targeting N-Myristoylation Through NMT2 Prevents Cardiac Hypertrophy and Heart Failure
- PMID: 38094695
- PMCID: PMC10714183
- DOI: 10.1016/j.jacbts.2023.06.006
Targeting N-Myristoylation Through NMT2 Prevents Cardiac Hypertrophy and Heart Failure
Abstract
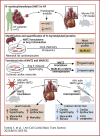
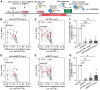
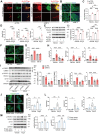
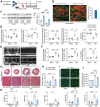
Protein diversity can increase via N-myristoylation, adding myristic acid to an N-terminal glycine residue. In a murine model of pressure overload, knockdown of cardiac N-myristoyltransferase 2 (NMT2) by adeno-associated virus 9 exacerbated cardiac dysfunction, remodeling, and failure. Click chemistry-based quantitative chemical proteomics identified substrate proteins of N-myristoylation in cardiac myocytes. N-myristoylation of MARCKS regulated angiotensin II-induced cardiac pathological hypertrophy by preventing activations of Ca2+/calmodulin-dependent protein kinase II and histone deacetylase 4 and histone acetylation. Gene transfer of NMT2 to the heart reduced cardiac dysfunction and failure, suggesting targeting N-myristoylation through NMT2 could be a potential therapeutic approach for preventing cardiac remodeling and heart failure.
Keywords: N-myristoylation; cardiac remodeling; gene therapy; heart failure; post-translational modifications.
© 2023 The Authors.
Conflict of interest statement
This work was supported by the Japan Society for the Promotion of Science KAKENHI grants 22K08161 (Dr Tomita), 20K22907 (Dr Anzai), and 19K17572 (Dr Misaka). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures






References
-
- Nakamura M., Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018;15(7):387–407. - PubMed
-
- Lundby A., Andersen M.N., Steffensen A.B., et al. In vivo phosphoproteomics analysis reveals the cardiac targets of beta-adrenergic receptor signaling. Sci Signal. 2013;6(278):rs11. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

