Calprotectin blockade inhibits long-term vascular pathology following peritoneal dialysis-associated bacterial infection
- PMID: 38094743
- PMCID: PMC10716465
- DOI: 10.3389/fcimb.2023.1285193
Calprotectin blockade inhibits long-term vascular pathology following peritoneal dialysis-associated bacterial infection
Abstract
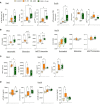
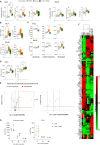
Bacterial infections and the concurrent inflammation have been associated with increased long-term cardiovascular (CV) risk. In patients receiving peritoneal dialysis (PD), bacterial peritonitis is a common occurrence, and each episode further increases late CV mortality risk. However, the underlying mechanism(s) remains to be elucidated before safe and efficient anti-inflammatory interventions can be developed. Damage-Associated Molecular Patterns (DAMPs) have been shown to contribute to the acute inflammatory response to infections, but a potential role for DAMPs in mediating long-term vascular inflammation and CV risk following infection resolution in PD, has not been investigated. We found that bacterial peritonitis in mice that resolved within 24h led to CV disease-promoting systemic and vascular immune-mediated inflammatory responses that were maintained up to 28 days. These included higher blood proportions of inflammatory leukocytes displaying increased adhesion molecule expression, higher plasma cytokines levels, and increased aortic inflammatory and atherosclerosis-associated gene expression. These effects were also observed in infected nephropathic mice and amplified in mice routinely exposed to PD fluids. A peritonitis episode resulted in elevated plasma levels of the DAMP Calprotectin, both in PD patients and mice, here the increase was maintained up to 28 days. In vitro, the ability of culture supernatants from infected cells to promote key inflammatory and atherosclerosis-associated cellular responses, such as monocyte chemotaxis, and foam cell formation, was Calprotectin-dependent. In vivo, Calprotectin blockade robustly inhibited the short and long-term peripheral and vascular consequences of peritonitis, thereby demonstrating that targeting of the DAMP Calprotectin is a promising therapeutic strategy to reduce the long-lasting vascular inflammatory aftermath of an infection, notably PD-associated peritonitis, ultimately lowering CV risk.
Keywords: anti-inflammatory intervention strategies; damage associated moeleular patterns; infection; peritoneal dialysis; vascular inflammation.
Copyright © 2023 Cetin, Mazzarino, González-Mateo, Kopytina, Meran, Fraser, López-Cabrera, Labéta and Raby.
Conflict of interest statement
Author GG-M was employed by Premium Research, S.L. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Amaya-Garrido A., Brunet M., Buffin-Meyer B., Piedrafita A., Grzesiak L., Agbegbo E., et al. (2023). Calprotectin is a contributor to and potential therapeutic target for vascular calcification in chronic kidney disease. Sci. Trans. Med. 15, eabn5939. doi: 10.1126/scitranslmed.abn5939 - DOI - PubMed
-
- Bengtsson A. A., Sturfelt G., Lood C., Ronnblom L., van Vollenhoven R. F., Axelsson B., et al. (2012). Pharmacokinetics, tolerability, and preliminary efficacy of paquinimod (ABR-215757), a new quinoline-3-carboxamide derivative: studies in lupus-prone mice and a multicenter, randomized, double-blind, placebo-controlled, repeat-dose, dose-ranging study in patients with systemic lupus erythematosus. Arthritis Rheum 64, 1579–1588. doi: 10.1002/art.33493 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

