Applicability of Redirecting Artemisinins for New Targets
- PMID: 38094863
- PMCID: PMC10714028
- DOI: 10.1002/gch2.202300030
Applicability of Redirecting Artemisinins for New Targets
Abstract
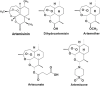
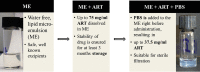
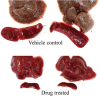
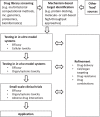
Employing new therapeutic indications for drugs that are already approved for human use has obvious advantages, including reduced costs and timelines, because some routine steps of drug development and regulation are not required. This work concentrates on the redirection of artemisinins (ARTS) that already are approved for clinical use, or investigated, for malaria treatment. Several mechanisms of action are suggested for ARTS, among which only a few have been successfully examined in vivo, mainly the induction of oxidant stress and anti-inflammatory effects. Despite these seemingly contradictory effects, ARTS are proposed for repurposing in treatment of inflammatory disorders and diverse types of diseases caused by viral, bacterial, fungal, and parasitic infections. When pathogens are treated the expected outcome is diminution of the causative agents and/or their inflammatory damage. In general, repurposing ARTS is successful in only a very few cases, specifically when a valid mechanism can be targeted using an additional therapeutic agent and appropriate drug delivery. Investigation of repurposing should include optimization of drug combinations followed by examination in relevant cell lines, organoids, and animal models, before moving to clinical trials.
Keywords: bacterial infections; cancer; microbiota; parasites; repurposing artemisinin; viral infections.
© 2023 The Authors. Global Challenges published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Scherman D., Fetro C., Therapie 2020, 75, 161. - PubMed
-
- Pushpakom S., Iorio F., Eyers P. A., Escott K. J., Hopper S., Wells A., Doig A., Guilliams T., Latimer J., Mcnamee C., Norris A., Sanseau P., Cavalla D., Pirmohamed M., Nat Rev Drug Discov 2019, 18, 41. - PubMed
-
- Tu Y., Nat. Med. 2011, 17, 1217. - PubMed
-
- Rasmussen C., Alonso P., Ringwald P., Expert Rev. Anti Infect. Ther. 2022, 20, 353. - PubMed
-
- WHO Guidelines for malaria , https://pubmed.ncbi.nlm.nih.gov/36445950/ (acessed: March 2022).
Publication types
LinkOut - more resources
Full Text Sources

