The Role of MAPRE2 and Microtubules in Maintaining Normal Ventricular Conduction
- PMID: 38095085
- PMCID: PMC11889334
- DOI: 10.1161/CIRCRESAHA.123.323231
The Role of MAPRE2 and Microtubules in Maintaining Normal Ventricular Conduction
Abstract
Background: Brugada syndrome is associated with loss-of-function SCN5A variants, yet these account for only ≈20% of cases. A recent genome-wide association study identified a novel locus within MAPRE2, which encodes EB2 (microtubule end-binding protein 2), implicating microtubule involvement in Brugada syndrome.
Methods: A mapre2 knockout zebrafish model was generated using CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats/clustered regularly interspaced short palindromic repeat-associated protein 9) and validated by Western blot. Larval hearts at 5 days post-fertilization were isolated for voltage mapping and immunocytochemistry. Adult fish hearts were used for ECG, patch clamping, and immunocytochemistry. Morpholinos were injected into embryos at 1-cell stage for knockdown experiments. A transgenic zebrafish line with cdh2 tandem fluorescent timer was used to study adherens junctions. Microtubule plus-end tracking and patch clamping were performed in human induced pluripotent stem cell derived cardiomyocytes (iPSC-CMs) with MAPRE2 knockdown and knockout, respectively.
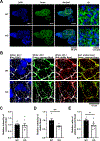
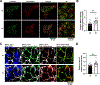
Results: Voltage mapping of mapre2 knockout hearts showed a decrease in ventricular maximum upstroke velocity of the action potential and conduction velocity, suggesting loss of cardiac voltage-gated sodium channel function. ECG showed QRS prolongation in adult knockout fish, and patch clamping showed decreased sodium current density in knockout ventricular myocytes and arrhythmias in knockout iPSC-CMs. Confocal imaging showed disorganized adherens junctions and mislocalization of mature Ncad (N-cadherin) with mapre2 loss of function, associated with a decrease of detyrosinated tubulin. MAPRE2 knockdown in iPSC-CMs led to an increase in microtubule growth velocity and distance, indicating changes in microtubule dynamics. Finally, knockdown of ttl encoding tubulin tyrosine ligase in mapre2 knockout larvae rescued tubulin detyrosination and ventricular maximum upstroke velocity of the action potential.
Conclusions: Genetic ablation of mapre2 led to a decrease in voltage-gated sodium channel function, a hallmark of Brugada syndrome, associated with disruption of adherens junctions, decrease of detyrosinated tubulin as a marker of microtubule stability, and changes in microtubule dynamics. Restoration of the detyrosinated tubulin fraction with ttl knockdown led to rescue of voltage-gated sodium channel-related functional parameters in mapre2 knockout hearts. Taken together, our study implicates microtubule dynamics in the modulation of ventricular conduction.
Keywords: arrhythmias, cardiac; electrophysiology; microtubules; sodium channels; zebrafish.
Conflict of interest statement
Figures

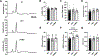
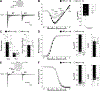


References
-
- Wilde AAM, Brugada R. Phenotypical manifestations of mutations in the genes encoding subunits of the cardiac sodium channel. Circ. Res [Internet]. 2011;108:884–97. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21454796 - PubMed
-
- Remme CA. Cardiac sodium channelopathy associated with SCN5A mutations: electrophysiological, molecular and genetic aspects. J. Physiol [Internet]. 2013;591:4099–116. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23818691 - PMC - PubMed
-
- Shimizu W. The Brugada syndrome--an update. Intern. Med [Internet]. 2005;44:1224–31. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16415541 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

