Haematopoietic development and HSC formation in vitro: promise and limitations of gastruloid models
- PMID: 38095554
- PMCID: PMC10754337
- DOI: 10.1042/ETLS20230091
Haematopoietic development and HSC formation in vitro: promise and limitations of gastruloid models
Abstract
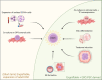
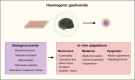
Haematopoietic stem cells (HSCs) are the most extensively studied adult stem cells. Yet, six decades after their first description, reproducible and translatable generation of HSC in vitro remains an unmet challenge. HSC production in vitro is confounded by the multi-stage nature of blood production during development. Specification of HSC is a late event in embryonic blood production and depends on physical and chemical cues which remain incompletely characterised. The precise molecular composition of the HSC themselves is incompletely understood, limiting approaches to track their origin in situ in the appropriate cellular, chemical and mechanical context. Embryonic material at the point of HSC emergence is limiting, highlighting the need for an in vitro model of embryonic haematopoietic development in which current knowledge gaps can be addressed and exploited to enable HSC production. Gastruloids are pluripotent stem cell-derived 3-dimensional (3D) cellular aggregates which recapitulate developmental events in gastrulation and early organogenesis with spatial and temporal precision. Gastruloids self-organise multi-tissue structures upon minimal and controlled external cues, and are amenable to live imaging, screening, scaling and physicochemical manipulation to understand and translate tissue formation. In this review, we consider the haematopoietic potential of gastruloids and review early strategies to enhance blood progenitor and HSC production. We highlight possible strategies to achieve HSC production from gastruloids, and discuss the potential of gastruloid systems in illuminating current knowledge gaps in HSC specification.
Keywords: gastruloid models; haematopoietic stem cells; pluripotent stem cells.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

Similar articles
-
Modeling post-implantation stages of human development into early organogenesis with stem-cell-derived peri-gastruloids.Cell. 2023 Aug 31;186(18):3776-3792.e16. doi: 10.1016/j.cell.2023.07.018. Epub 2023 Jul 20. Cell. 2023. PMID: 37478861
-
Gastruloids: Pluripotent stem cell models of mammalian gastrulation and embryo engineering.Dev Biol. 2022 Aug;488:35-46. doi: 10.1016/j.ydbio.2022.05.002. Epub 2022 May 7. Dev Biol. 2022. PMID: 35537519 Free PMC article.
-
3D gastruloids: a novel frontier in stem cell-based in vitro modeling of mammalian gastrulation.Trends Cell Biol. 2021 Sep;31(9):747-759. doi: 10.1016/j.tcb.2021.06.007. Epub 2021 Jul 22. Trends Cell Biol. 2021. PMID: 34304959 Review.
-
Human haematopoietic stem cell development: from the embryo to the dish.Development. 2017 Jul 1;144(13):2323-2337. doi: 10.1242/dev.134866. Development. 2017. PMID: 28676567 Review.
-
Mapping human haematopoietic stem cells from haemogenic endothelium to birth.Nature. 2022 Apr;604(7906):534-540. doi: 10.1038/s41586-022-04571-x. Epub 2022 Apr 13. Nature. 2022. PMID: 35418685 Free PMC article.
References
-
- Fujimoto, T., Ogawa, M., Minegishi, N., Yoshida, H., Yokomizo, T., Yamamoto, M.et al. (2001) Step-wise divergence of primitive and definitive haematopoietic and endothelial cell lineages during embryonic stem cell differentiation. Genes Cells 6, 1113–1127 10.1046/j.1365-2443.2001.00490.x - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
