Dose-response effects on HbA1c and bodyweight reduction of survodutide, a dual glucagon/GLP-1 receptor agonist, compared with placebo and open-label semaglutide in people with type 2 diabetes: a randomised clinical trial
- PMID: 38095657
- PMCID: PMC10844353
- DOI: 10.1007/s00125-023-06053-9
Dose-response effects on HbA1c and bodyweight reduction of survodutide, a dual glucagon/GLP-1 receptor agonist, compared with placebo and open-label semaglutide in people with type 2 diabetes: a randomised clinical trial
Erratum in
-
Correction to: Dose-response effects on HbA1c and bodyweight reduction of survodutide, a dual glucagon/GLP-1 receptor agonist, compared with placebo and open-label semaglutide in people with type 2 diabetes: a randomised clinical trial.Diabetologia. 2024 Apr;67(4):758. doi: 10.1007/s00125-024-06095-7. Diabetologia. 2024. PMID: 38349400 Free PMC article. No abstract available.
Abstract
Aims/hypothesis: The aim of this study was to assess the dose-response effects of the subcutaneous glucagon receptor/glucagon-like peptide-1 receptor dual agonist survodutide (BI 456906) on HbA1c levels and bodyweight reduction.
Methods: This Phase II, multicentre, randomised, double-blind, parallel-group, placebo-controlled study, conducted in clinical research centres, assessed survodutide in participants aged 18-75 years with type 2 diabetes, an HbA1c level of 53-86 mmol/mol (7.0-10.0%) and a BMI of 25-50 kg/m2 on a background of metformin therapy. Participants were randomised via interactive response technology to receive survodutide (up to 0.3, 0.9, 1.8 or 2.7 mg once weekly [qw; dose group (DG) 1-4, respectively] or 1.2 or 1.8 mg twice weekly [DG 5 and 6, respectively]), placebo or semaglutide (up to 1.0 mg qw). Participants and all those involved in the trial conduct/analysis were blinded; the semaglutide arm was open-label. The primary endpoint was absolute change from baseline in HbA1c after 16 weeks' treatment. The key secondary endpoint was relative change from baseline in bodyweight after 16 weeks' treatment.
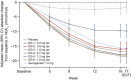
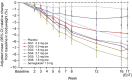
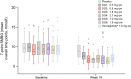
Results: A total of 413 participants were randomised (DG1, n=50; DG2, n=50; DG3, n=52; DG4, n=50; DG5, n=51; DG6, n=50; semaglutide, n=50; placebo, n=60). The full analysis set comprised 411 treated participants (DG6, n=49; placebo, n=59). Adjusted mean (95% CI) HbA1c decreased from baseline (mean ± SD 64.7±9.2 mmol/mol [8.07±0.84%] after 16 weeks' treatment: DG1 (n=41), -9.92 mmol/mol (-12.27, -7.56; -0.91% [-1.12, -0.69]); DG2 (n=46), -15.95 mmol/mol (-18.27, -13.63; -1.46% [-1.67, -1.25]); DG3 (n=36), -18.72 mmol/mol (-21.15, -16.29; -1.71% [-1.94, -1.49]); DG4 (n=33), -17.01 mmol/mol (-19.59, -14.43; -1.56% [-1.79, -1.32]); DG5 (n=44), -17.84 mmol/mol (-20.18, -15.51; -1.63% [-1.85, -1.42]); DG6 (n=36), -18.38 mmol/mol (-20.90, -15.87; -1.68% [-1.91, -1.45]). The mean reduction in HbA1c was similar with low-dose survodutide (DG2: -15.95 mmol/mol [-1.46%]; n=46) and semaglutide (-16.07 mmol/mol [-1.47%]; n=45). Mean (95% CI) bodyweight decreased dose-dependently up to -8.7% (-10.1, -7.3; DG6, n=37); survodutide ≥1.8 mg qw produced greater bodyweight reductions than semaglutide (-5.3% [-6.6, -4.1]; n=45). Adverse events (AEs) were reported for 77.8% of survodutide-treated participants (mainly gastrointestinal), 52.5% receiving placebo and 52.0% receiving semaglutide.
Conclusions/interpretation: Survodutide reduced HbA1c levels and bodyweight after 16 weeks' treatment in participants with type 2 diabetes. Dose-related gastrointestinal AEs could be mitigated with slower dose escalations.
Trial registration: ClinicalTrials.gov NCT04153929 and EudraCT 2019-002390-60.
Funding: Boehringer Ingelheim Pharma GmbH & Co. KG, Ingelheim, Germany.
Keywords: Bodyweight loss; Dual incretin agonist; Glucagon; Glucagon-like peptide-1; Obesity; Pharmacotherapy; Semaglutide; Survodutide; Type 2 diabetes.
© 2023. The Author(s).
Figures
References
-
- Pi-Sunyer X, Astrup A, Fujioka K et al (2015) A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 373(1):11-22. 10.1056/NEJMoa141189210.1056/NEJMoa1411892 - PubMed
-
- Liraglutide (Victoza) [package insert] (2023). Novo Nordisk A/S. Available from: https://www.novo-pi.com/victoza.pdf. Accessed 7 Jul 2023
-
- Semaglutide (Ozempic) [package insert] (2022). Novo Nordisk A/S. Available from: https://www.novo-pi.com/ozempic.pdf. Accessed 7 Jul 2023
-
- Dulaglutide (Trulicity) [package insert] (2022). Eli Lilly. Available from: https://pi.lilly.com/us/trulicity-uspi.pdf. Accessed 7 Jul 2023
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

