Pembrolizumab or Placebo Plus Chemotherapy With or Without Bevacizumab for Persistent, Recurrent, or Metastatic Cervical Cancer: Subgroup Analyses From the KEYNOTE-826 Randomized Clinical Trial
- PMID: 38095881
- PMCID: PMC10722390
- DOI: 10.1001/jamaoncol.2023.5410
Pembrolizumab or Placebo Plus Chemotherapy With or Without Bevacizumab for Persistent, Recurrent, or Metastatic Cervical Cancer: Subgroup Analyses From the KEYNOTE-826 Randomized Clinical Trial
Abstract
Importance: The KEYNOTE-826 randomized clinical trial showed statistically significant and clinically meaningful survival benefits with the addition of pembrolizumab to chemotherapy with or without bevacizumab in patients with persistent, recurrent, or metastatic cervical cancer. Treatment effects in patient subgroups of the study population are unknown.
Objective: To assess efficacy outcomes in patient subgroups of KEYNOTE-826.
Design, setting, and participants: Exploratory subgroup analyses were conducted in a global, phase 3, randomized, double-blind, placebo-controlled clinical trial. Participants included women with persistent, recurrent, or metastatic adenocarcinoma, adenosquamous carcinoma, or squamous cell carcinoma of the cervix that had not been treated with systemic chemotherapy and was not amenable to curative treatment. This subanalysis was conducted from November 20, 2018, to May 3, 2021.
Interventions: Pembrolizumab, 200 mg, every 3 weeks or placebo for up to 35 cycles plus chemotherapy (paclitaxel, 175 mg/m2, plus cisplatin, 50 mg/m2, or carboplatin AUC 5 [area under the free carboplatin plasma concentration vs time curve]) with or without bevacizumab, 15 mg/kg.
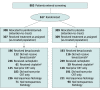
Main outcomes and measures: Overall survival (OS) and progression-free survival (PFS) by investigator assessment per Response Evaluation Criteria in Solid Tumors version 1.1 in subgroups defined by use of bevacizumab (yes or no), choice of platinum (carboplatin or cisplatin), prior chemoradiotherapy (CRT) exposure only (yes or no), and histologic type (squamous or nonsquamous) in patients with programmed cell death ligand 1-positive tumors (defined as a combined positive score [CPS] ≥1) and in the intention-to-treat population.
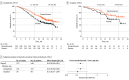
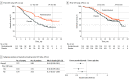
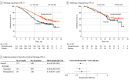
Results: A total of 617 patients (median age, 51 years; range, 22-82 years) were enrolled in the trial. In the CPS greater than or equal to 1 population, hazard ratios (HRs) for OS favored the pembrolizumab group in all subgroups: with bevacizumab (HR, 0.62; 95% CI, 0.45-0.87) and without bevacizumab (HR, 0.67; 95% CI, 0.47-0.96), use of carboplatin (HR, 0.65; 95% CI, 0.50-0.85) and cisplatin (HR, 0.53; 95% CI, 0.27-1.04), with prior CRT only (HR, 0.56; 95% CI, 0.39-0.81) and without prior CRT only (HR, 0.72; 95% CI, 0.52-1.00), and squamous (HR, 0.60; 95% CI, 0.46-0.79) and nonsquamous (HR, 0.70; 95% CI, 0.41-1.20) histologic type. In the intention-to-treat population, HRs for OS also favored the pembrolizumab group in all subgroups: with bevacizumab (HR, 0.63; 95% CI, 0.47-0.87) and without bevacizumab (HR, 0.74; 95% CI, 0.53-1.04), use of carboplatin (HR, 0.69; 95% CI, 0.54-0.89) or cisplatin (HR, 0.59; 95% CI, 0.32-1.09), with prior CRT only (HR, 0.64; 95% CI, 0.45-0.91) and without prior CRT only (HR, 0.71; 95% CI, 0.53-0.97), and squamous (HR, 0.61; 95% CI, 0.47-0.80) and nonsquamous (HR, 0.76; 95% CI, 0.47-1.23) histologic type. Similar to OS, the addition of pembrolizumab prolonged PFS across all subgroups in the CPS greater than or equal to 1 and intention-to-treat populations.
Conclusions and relevance: The findings of this trial suggest that adding pembrolizumab to chemotherapy with or without bevacizumab improved OS across subgroups of patients with persistent, recurrent, or metastatic cervical cancer.
Trial registration: ClinicalTrials.gov Identifier: NCT03635567.
Conflict of interest statement
Figures

References
-
- International Agency for Research on Cancer, World Health Organization . Estimated number of incident cases worldwide, both sexes, all ages. Cancer Today. 2020. Accessed June 1, 2022. https://gco.iarc.fr/today/home
-
- International Agency for Research on Cancer, World Health Organization . Estimated number of deaths in 2020, worldwide, females, all ages. Cancer Today. 2020. Accessed June 1, 2022. https://gco.iarc.fr/today/home
-
- International Agency for Research on Cancer, World Health Organization . Estimated number of new cases in 2020, worldwide, females, all ages. Cancer Today. 2020. Accessed June 1, 2022. https://gco.iarc.fr/today/home
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

