Hemodialysis Access: US for Preprocedural Mapping and Evaluation of Maturity and Access Dysfunction
- PMID: 38096113
- PMCID: PMC10772307
- DOI: 10.1148/rg.230053
Hemodialysis Access: US for Preprocedural Mapping and Evaluation of Maturity and Access Dysfunction
Abstract
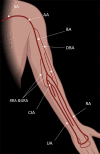
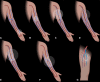
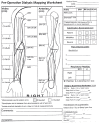
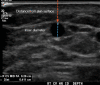
Patients with kidney failure require kidney replacement therapy. While renal transplantation remains the treatment of choice for kidney failure, renal replacement therapy with hemodialysis may be required owing to the limited availability and length of time patients may wait for allografts or for patients ineligible for transplant owing to advanced age or comorbidities. The ideal hemodialysis access should provide complication-free dialysis by creating a direct connection between an artery and vein with adequate blood flow that can be reliably and easily accessed percutaneously several times a week. Surgical arteriovenous fistulas and grafts are commonly created for hemodialysis access, with newer techniques that involve the use of minimally invasive endovascular approaches. The emphasis on proactive planning for the placement, protection, and preservation of the next vascular access before the current one fails has increased the use of US for preoperative mapping and monitoring of complications for potential interventions. Preoperative US of the extremity vasculature helps assess anatomic suitability before vascular access creation, increasing the rates of successful maturation. A US mapping protocol ensures reliable measurements and clear communication of anatomic variants that may alter surgical planning. Postoperative imaging helps assess fistula maturation before cannulation for dialysis and evaluates for early and late complications associated with arteriovenous access. Clinical and US findings can suggest developing stenosis that may progress to thrombosis and loss of access function, which can be treated with percutaneous vascular interventions to preserve access patency. Vascular access steal, aneurysms and pseudoaneurysms, and fluid collections are other complications amenable to US evaluation. ©RSNA, 2023 Supplemental material is available for this article. Test Your Knowledge questions for this article are available through the Online Learning Center.
Conflict of interest statement
Figures

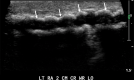

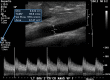

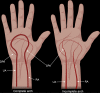

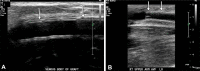
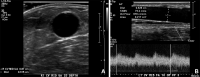




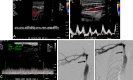
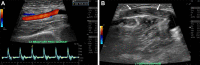
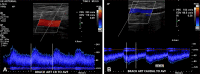
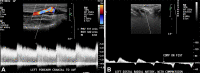

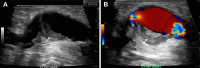
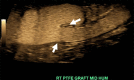
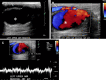

References
-
- United States Renal Data System . USRDS Annual Data Report: Epidemiology of kidney disease in the United States . Bethesda, MD: : National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; , 2022. .
-
- Jager KJ , Kovesdy C , Langham R , Rosenberg M , Jha V , Zoccali C . A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases . Nephrol Dial Transplant 2019. ; 34 ( 11 ): 1803 – 1805 . - PubMed
-
- Lok CE , Huber TS , Lee T , et al. ; National Kidney Foundation . KDOQI Clinical Practice Guideline for Vascular Access: 2019 Update . Am J Kidney Dis 2020. ; 75 ( suppl 2 ): S1 – S164 [Published correction appears in Am J Kidney Dis 2021;77(4):551.]. - PubMed
-
- Liyanage T , Ninomiya T , Jha V , et al. . Worldwide access to treatment for end-stage kidney disease: a systematic review . Lancet 2015. ; 385 ( 9981 ): 1975 – 1982 . - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

