Microbiome diversity protects against pathogens by nutrient blocking
- PMID: 38096285
- PMCID: PMC7616675
- DOI: 10.1126/science.adj3502
Microbiome diversity protects against pathogens by nutrient blocking
Abstract
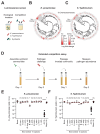
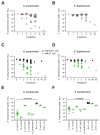
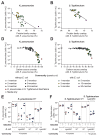
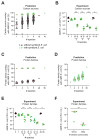
The human gut microbiome plays an important role in resisting colonization of the host by pathogens, but we lack the ability to predict which communities will be protective. We studied how human gut bacteria influence colonization of two major bacterial pathogens, both in vitro and in gnotobiotic mice. Whereas single species alone had negligible effects, colonization resistance greatly increased with community diversity. Moreover, this community-level resistance rested critically upon certain species being present. We explained these ecological patterns through the collective ability of resistant communities to consume nutrients that overlap with those used by the pathogen. Furthermore, we applied our findings to successfully predict communities that resist a novel target strain. Our work provides a reason why microbiome diversity is beneficial and suggests a route for the rational design of pathogen-resistant communities.
Conflict of interest statement
Figures

References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

