Tricuspid valve disease and cardiac implantable electronic devices
- PMID: 38096587
- PMCID: PMC10834167
- DOI: 10.1093/eurheartj/ehad783
Tricuspid valve disease and cardiac implantable electronic devices
Erratum in
-
Correction to: Tricuspid valve disease and cardiac implantable electronic devices.Eur Heart J. 2024 May 13;45(18):1680. doi: 10.1093/eurheartj/ehae221. Eur Heart J. 2024. PMID: 38552211 Free PMC article. No abstract available.
Abstract
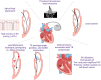
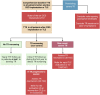
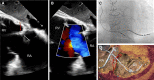
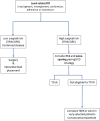
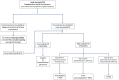
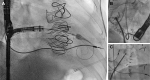
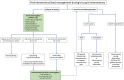
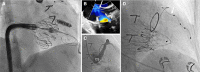
The role of cardiac implantable electronic device (CIED)-related tricuspid regurgitation (TR) is increasingly recognized as an independent clinical entity. Hence, interventional TR treatment options continuously evolve, surgical risk assessment and peri-operative care improve the management of CIED-related TR, and the role of lead extraction is of high interest. Furthermore, novel surgical and interventional tricuspid valve treatment options are increasingly applied to patients suffering from TR associated with or related to CIEDs. This multidisciplinary review article developed with electrophysiologists, interventional cardiologists, imaging specialists, and cardiac surgeons aims to give an overview of the mechanisms of disease, diagnostics, and proposes treatment algorithms of patients suffering from TR associated with CIED lead(s) or leadless pacemakers.
Keywords: Cardiac implantable electronic device; Lead related; Pacemaker; Tricuspid regurgitation.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures



References
-
- Raatikainen MJ, Arnar DO, Zeppenfeld K, Merino JL, Levya F, Hindriks G, et al. Statistics on the use of cardiac electronic devices and electrophysiological procedures in the European Society of Cardiology countries: 2014 report from the European Heart Rhythm Association. Europace 2015;17:i1–75. 10.1093/europace/euu300 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

