Luteolin prevents TNF-α-induced NF-κB activation and ROS production in cultured human placental explants and endothelial cells
- PMID: 38096686
- PMCID: PMC10872317
- DOI: 10.1016/j.placenta.2023.12.004
Luteolin prevents TNF-α-induced NF-κB activation and ROS production in cultured human placental explants and endothelial cells
Abstract
Introduction: Preeclampsia (PE) is a serious hypertensive pregnancy disorder and a leading cause of maternal and perinatal morbidity and mortality. Despite the prevalence and complications, there are no approved therapeutics to relieve PE symptoms. Inflammation, oxidative stress, and angiogenic imbalance have been shown to contribute to the PE pathophysiology, though there is a lack of understanding in how best to target these pathways in PE. We recently demonstrated that the bioflavonoid luteolin is a potent inhibitor of the anti-angiogenic and pro-hypertensive soluble fms-like tyrosine kinase 1 (sFlt-1), and here we aimed to determine if luteolin was also capable of reducing inflammation and oxidative stress pathways.
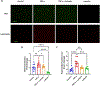
Methods: Tumor necrosis factor (TNF)-α, which is upregulated in PE, was utilized to stimulate these pathways in human placental explants and endothelial cells. Endothelin-1 (ET-1) and interleukin (IL)-6 in the media from explants and cells were measured via ELISA, and NF-κB localization and reactive oxygen species were detected via fluorescence microscopy.
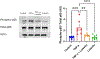
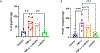
Results: Pretreatment with luteolin demonstrated significant reductions in NF-κB activation, reactive oxygen species, superoxide, and IL-6 and ET-1 expression in endothelial cells. We also saw a significant reduction in phosphorylation of NF-κB in human placental explants.
Discussion: These data demonstrate that luteolin inhibits pathways implicated in the development of PE and should be explored further for its potential as a PE therapeutic.
Keywords: ET-1; Inflammation; Luteolin; NF-κB p65; Preeclampsia; ROS.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest SR reports serving as a consultant for Roche Diagnostics, ThermoFisher, Siemens and has received research funding from Roche Diagnostics and Siemens for work related to angiogenic biomarkers unrelated to this work. All other authors have no conflict of interest.
Figures

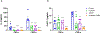
Similar articles
-
Bioflavonoid luteolin prevents sFlt-1 release via HIF-1α inhibition in cultured human placenta.FASEB J. 2023 Aug;37(8):e23078. doi: 10.1096/fj.202300611R. FASEB J. 2023. PMID: 37405762 Free PMC article.
-
Luteolin protects HUVECs from TNF-α-induced oxidative stress and inflammation via its effects on the Nox4/ROS-NF-κB and MAPK pathways.J Atheroscler Thromb. 2014;21(8):768-83. doi: 10.5551/jat.23697. Epub 2014 Mar 12. J Atheroscler Thromb. 2014. PMID: 24621786
-
Mechanism of astragalus injection to relieve symptoms of preeclampsia rat model by inhibiting MMP-9/sFlt-1/TNF-α.Altern Ther Health Med. 2023 Mar;29(2):125-131. Altern Ther Health Med. 2023. PMID: 36399080
-
Pregnancy-specific expression of protease-activated receptor 1: a therapeutic target for prevention and treatment of preeclampsia?Am J Obstet Gynecol. 2022 Feb;226(2S):S945-S953. doi: 10.1016/j.ajog.2021.11.1367. Am J Obstet Gynecol. 2022. PMID: 35177224 Free PMC article. Review.
-
Increased risk for the development of preeclampsia in obese pregnancies: weighing in on the mechanisms.Am J Physiol Regul Integr Comp Physiol. 2015 Dec 1;309(11):R1326-43. doi: 10.1152/ajpregu.00178.2015. Epub 2015 Oct 7. Am J Physiol Regul Integr Comp Physiol. 2015. PMID: 26447211 Free PMC article. Review.
Cited by
-
Recent insights into luteolin and its biological and pharmacological activities.EXCLI J. 2024 May 16;23:787-794. doi: 10.17179/excli2024-7168. eCollection 2024. EXCLI J. 2024. PMID: 39165588 Free PMC article. No abstract available.
References
-
- ACOG Practice Bulletin No. 202 Summary: Gestational Hypertension and Preeclampsia. Obstet Gynecol, 2019. 133(1): p. 1. - PubMed
-
- Bates DO, Pre-eclampsia and the microcirculation: a novel explanation. Clin Sci (Lond), 2003. 104(4): p. 413–4. - PubMed
-
- Zárate A, et al., Early disturbed placental ischemia and hypoxia creates immune alteration and vascular disorder causing preeclampsia. Arch Med Res, 2014. 45(7): p. 519–24. - PubMed
-
- Lee JI and Burckart GJ, Nuclear factor kappa B: important transcription factor and therapeutic target. The Journal of Clinical Pharmacology, 1998. 38(11): p. 981–993. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

