Structures, functions and adaptations of the human LINE-1 ORF2 protein
- PMID: 38096902
- PMCID: PMC10830420
- DOI: 10.1038/s41586-023-06947-z
Structures, functions and adaptations of the human LINE-1 ORF2 protein
Abstract
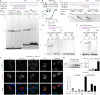
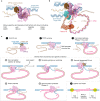
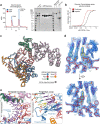
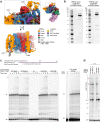
The LINE-1 (L1) retrotransposon is an ancient genetic parasite that has written around one-third of the human genome through a 'copy and paste' mechanism catalysed by its multifunctional enzyme, open reading frame 2 protein (ORF2p)1. ORF2p reverse transcriptase (RT) and endonuclease activities have been implicated in the pathophysiology of cancer2,3, autoimmunity4,5 and ageing6,7, making ORF2p a potential therapeutic target. However, a lack of structural and mechanistic knowledge has hampered efforts to rationally exploit it. We report structures of the human ORF2p 'core' (residues 238-1061, including the RT domain) by X-ray crystallography and cryo-electron microscopy in several conformational states. Our analyses identified two previously undescribed folded domains, extensive contacts to RNA templates and associated adaptations that contribute to unique aspects of the L1 replication cycle. Computed integrative structural models of full-length ORF2p show a dynamic closed-ring conformation that appears to open during retrotransposition. We characterize ORF2p RT inhibition and reveal its underlying structural basis. Imaging and biochemistry show that non-canonical cytosolic ORF2p RT activity can produce RNA:DNA hybrids, activating innate immune signalling through cGAS/STING and resulting in interferon production6-8. In contrast to retroviral RTs, L1 RT is efficiently primed by short RNAs and hairpins, which probably explains cytosolic priming. Other biochemical activities including processivity, DNA-directed polymerization, non-templated base addition and template switching together allow us to propose a revised L1 insertion model. Finally, our evolutionary analysis demonstrates structural conservation between ORF2p and other RNA- and DNA-dependent polymerases. We therefore provide key mechanistic insights into L1 polymerization and insertion, shed light on the evolutionary history of L1 and enable rational drug development targeting L1.
© 2023. The Author(s).
Conflict of interest statement
M.S.T., B.D.G., M.G., K.H.B. and E.A. hold equity in and have received consulting fees from ROME Therapeutics. J.L. holds equity in ROME Therapeutics. D.H. and E.T. have received consulting fees from ROME Therapeutics. Research conducted at Proteros Biostructures and Charles River Laboratory was contracted by ROME Therapeutics. Research for this project in the Götte laboratory was sponsored by ROME Therapeutics. M.S.T. has received consulting fees from Tessera Therapeutics. K.H.B. declares relationships with Alamar Biosciences, Genscript, Oncolinea/PrimeFour Therapeutics, Scaffold Therapeutics, Tessera Therapeutics and Transposon Therapeutics.
Figures





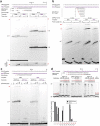
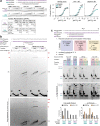
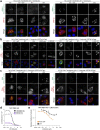


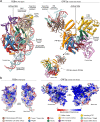
References
MeSH terms
Substances
Grants and funding
- R01 CA240924/CA/NCI NIH HHS/United States
- R01 AI081848/AI/NIAID NIH HHS/United States
- R01 AI027690/AI/NIAID NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- R01 GM126170/GM/NIGMS NIH HHS/United States
- T32 CA009216/CA/NCI NIH HHS/United States
- R01 AG078925/AG/NIA NIH HHS/United States
- P41 GM109824/GM/NIGMS NIH HHS/United States
- R01 GM130680/GM/NIGMS NIH HHS/United States
- K08 DK129824/DK/NIDDK NIH HHS/United States
- R01 GM083960/GM/NIGMS NIH HHS/United States
- T32 GM149436/GM/NIGMS NIH HHS/United States
- P01 AG051449/AG/NIA NIH HHS/United States
- U01 CA228963/CA/NCI NIH HHS/United States
- U24 GM129539/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

