Mucosal boosting enhances vaccine protection against SARS-CoV-2 in macaques
- PMID: 38096903
- PMCID: PMC10849944
- DOI: 10.1038/s41586-023-06951-3
Mucosal boosting enhances vaccine protection against SARS-CoV-2 in macaques
Abstract
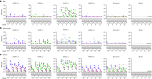
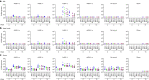
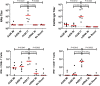
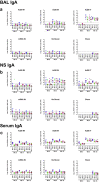
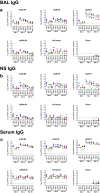
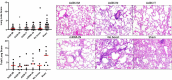
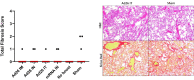
A limitation of current SARS-CoV-2 vaccines is that they provide minimal protection against infection with current Omicron subvariants1,2, although they still provide protection against severe disease. Enhanced mucosal immunity may be required to block infection and onward transmission. Intranasal administration of current vaccines has proven inconsistent3-7, suggesting that alternative immunization strategies may be required. Here we show that intratracheal boosting with a bivalent Ad26-based SARS-CoV-2 vaccine results in substantial induction of mucosal humoral and cellular immunity and near-complete protection against SARS-CoV-2 BQ.1.1 challenge. A total of 40 previously immunized rhesus macaques were boosted with a bivalent Ad26 vaccine by the intramuscular, intranasal and intratracheal routes, or with a bivalent mRNA vaccine by the intranasal route. Ad26 boosting by the intratracheal route led to a substantial expansion of mucosal neutralizing antibodies, IgG and IgA binding antibodies, and CD8+ and CD4+ T cell responses, which exceeded those induced by Ad26 boosting by the intramuscular and intranasal routes. Intratracheal Ad26 boosting also led to robust upregulation of cytokine, natural killer, and T and B cell pathways in the lungs. After challenge with a high dose of SARS-CoV-2 BQ.1.1, intratracheal Ad26 boosting provided near-complete protection, whereas the other boosting strategies proved less effective. Protective efficacy correlated best with mucosal humoral and cellular immune responses. These data demonstrate that these immunization strategies induce robust mucosal immunity, suggesting the feasibility of developing vaccines that block respiratory viral infections.
© 2023. The Author(s).
Conflict of interest statement
D.H.B., F.W. and R.C.Z. are listed as co-inventors on provisional SARS-CoV-2 vaccine patents (63/121,482; 63/133,969; 63/135,182). F.W., J.T., J.S., L.S., L.M.M.C. and R.C.Z. are employees and may hold equity in Janssen. The other authors declare no competing interests.
Figures









References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

