Genome-wide association study of preserved ratio impaired spirometry (PRISm)
- PMID: 38097206
- PMCID: PMC10765494
- DOI: 10.1183/13993003.00337-2023
Genome-wide association study of preserved ratio impaired spirometry (PRISm)
Abstract
Background: Preserved ratio impaired spirometry (PRISm) is defined as a forced expiratory volume in 1 s (FEV1) <80% predicted and FEV1/forced vital capacity ≥0.70. PRISm is associated with respiratory symptoms and comorbidities. Our objective was to discover novel genetic signals for PRISm and see if they provide insight into the pathogenesis of PRISm and associated comorbidities.
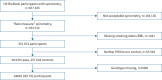
Methods: We undertook a genome-wide association study (GWAS) of PRISm in UK Biobank participants (Stage 1), and selected single nucleotide polymorphisms (SNPs) reaching genome-wide significance for replication in 13 cohorts (Stage 2). A combined meta-analysis of Stage 1 and Stage 2 was done to determine top SNPs. We used cross-trait linkage disequilibrium score regression to estimate genome-wide genetic correlation between PRISm and pulmonary and extrapulmonary traits. Phenome-wide association studies of top SNPs were performed.
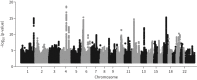
Results: 22 signals reached significance in the joint meta-analysis, including four signals novel for lung function. A strong genome-wide genetic correlation (rg) between PRISm and spirometric COPD (rg=0.62, p<0.001) was observed, and genetic correlation with type 2 diabetes (rg=0.12, p=0.007). Phenome-wide association studies showed that 18 of 22 signals were associated with diabetic traits and seven with blood pressure traits.
Conclusion: This is the first GWAS to successfully identify SNPs associated with PRISm. Four of the signals, rs7652391 (nearest gene MECOM), rs9431040 (HLX), rs62018863 (TMEM114) and rs185937162 (HLA-B), have not been described in association with lung function before, demonstrating the utility of using different lung function phenotypes in GWAS. Genetic factors associated with PRISm are strongly correlated with risk of both other lung diseases and extrapulmonary comorbidity.
The content of this work is not subject to copyright. Design and branding are copyright ©ERS 2024.
Conflict of interest statement
Conflict of interest: S.J. London reports support for the present work from National Institutes of Health (NIH), USA. M.H. Cho reports support for the present work from the National Heart, Lung, and Blood Institute (NHLBI) and grants from Bayer outside the submitted work. E. Wan reports grants from US Department of Veterans Affairs Research and Development, outside the submitted work. E. Silverman reports support for the present work from NIH (grant U01 HL089856) and has received institutional grant support from Bayer outside the submitted work. J.D. Crapo reports grants from NIH (U01 HL089897, U01 HL089856); and a leadership role as Chair of Board of Directors of COPD Foundation outside the submitted work. X. Hu reports support for the present manuscript from NIH/NHLBI (R01-HL153248), as well as travel support. L. Lahousse reports consulting fees from AstraZeneca and lecture honoraria from IPSA vzw and Chiesi, outside the submitted work. D.D. Sin reports lecture honoraria from GlaxoSmithKline, AstraZeneca and Boehringer Ingelheim, outside the submitted work. S.E. Harris reports support for the current manuscript from Biotechnology and Biological Sciences Research Council, and the Economic and Social Research Council (BB/W008793/1). M.D. Tobin, A.L. Guyatt and C. John have a funded research collaboration with Orion for collaborative research projects outside the submitted work. G. Davey Smith reports grants from MRC Integrative Epidemiology Unit at the University of Bristol (MC_UU_00011/1) and Scientific Advisory Board Membership for Relation Therapeutics and Insitro, outside the submitted work. M.D. Tobin reports support for the present work from Wellcome Trust, NIHR Leicester Biomedical Research Centre and National Institute of Health and Care Research, and has previously received funding from GlaxoSmithKline for collaborative research projects outside of the submitted work. J.W. Dodd declares he has received honoraria for participating in advisory boards and given lectures at meetings supported by GlaxoSmithKline, Boehringer Ingelheim, Chiesi, AstraZeneca, Fisher & Paykel and Aerogen; he has received sponsorship for attending international scientific meetings from Chiesi; he has also taken part in asthma clinical trials sponsored by Sanofi, AstraZeneca and Chiesi for which his institution received remuneration. All other authors have nothing to disclose.
Figures

Comment in
-
Some future directions for genome-wide association studies of preserved ratio impaired spirometry.Eur Respir J. 2024 Mar 7;63(3):2400142. doi: 10.1183/13993003.00142-2024. Print 2024 Mar. Eur Respir J. 2024. PMID: 38453246 No abstract available.
References
Publication types
MeSH terms
Grants and funding
- U01 HL080295/HL/NHLBI NIH HHS/United States
- U01 HL130114/HL/NHLBI NIH HHS/United States
- MC_UU_00032/1/MRC_/Medical Research Council/United Kingdom
- N01 HC095166/HL/NHLBI NIH HHS/United States
- N01 HC085081/HL/NHLBI NIH HHS/United States
- N01 HC095160/HL/NHLBI NIH HHS/United States
- N01 HC085080/HL/NHLBI NIH HHS/United States
- HHSN268201500003C/HL/NHLBI NIH HHS/United States
- R01 HL103612/HL/NHLBI NIH HHS/United States
- 75N92020D00002/HL/NHLBI NIH HHS/United States
- N01 HC095161/HL/NHLBI NIH HHS/United States
- 75N92020D00005/HL/NHLBI NIH HHS/United States
- N01 HC095168/HL/NHLBI NIH HHS/United States
- R01 HL120393/HL/NHLBI NIH HHS/United States
- UL1 TR001079/TR/NCATS NIH HHS/United States
- N02 HL064278/HL/NHLBI NIH HHS/United States
- N01 HC095169/HL/NHLBI NIH HHS/United States
- N01 HC085082/HL/NHLBI NIH HHS/United States
- HHSN268200800007C/HL/NHLBI NIH HHS/United States
- N01 HC085086/HL/NHLBI NIH HHS/United States
- 75N92020D00001/HL/NHLBI NIH HHS/United States
- N01 HC085083/HL/NHLBI NIH HHS/United States
- R01 HL153248/HL/NHLBI NIH HHS/United States
- U01 HL089897/HL/NHLBI NIH HHS/United States
- R01 HL077612/HL/NHLBI NIH HHS/United States
- MC_UU_00011/1/MRC_/Medical Research Council/United Kingdom
- N01 HC095167/HL/NHLBI NIH HHS/United States
- R01 HL087652/HL/NHLBI NIH HHS/United States
- Z01 ES049030/ImNIH/Intramural NIH HHS/United States
- R01 HL089856/HL/NHLBI NIH HHS/United States
- N01 HC095159/HL/NHLBI NIH HHS/United States
- 75N92020D00003/HL/NHLBI NIH HHS/United States
- R01 HL093081/HL/NHLBI NIH HHS/United States
- R01 HL105756/HL/NHLBI NIH HHS/United States
- U01 HL089856/HL/NHLBI NIH HHS/United States
- P30 DK063491/DK/NIDDK NIH HHS/United States
- N01 ES055546/ES/NIEHS NIH HHS/United States
- HHSN268201200036C/HL/NHLBI NIH HHS/United States
- HHSN268201800001C/HL/NHLBI NIH HHS/United States
- N01 HC055222/HL/NHLBI NIH HHS/United States
- MC_PC_19004/MRC_/Medical Research Council/United Kingdom
- R01 HL142028/HL/NHLBI NIH HHS/United States
- N01 HC085079/HL/NHLBI NIH HHS/United States
- UL1 TR001420/TR/NCATS NIH HHS/United States
- 75N92020D00004/HL/NHLBI NIH HHS/United States
- N01 HC095163/HL/NHLBI NIH HHS/United States
- 75N92020D00007/HL/NHLBI NIH HHS/United States
- Z01 ES043012/ImNIH/Intramural NIH HHS/United States
- HHSN268201500003I/HL/NHLBI NIH HHS/United States
- 75N92021D00006/HL/NHLBI NIH HHS/United States
- Z01 CP010119/ImNIH/Intramural NIH HHS/United States
- G0902313/MRC_/Medical Research Council/United Kingdom
- UL1 TR000040/TR/NCATS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- N01 HC095162/HL/NHLBI NIH HHS/United States
- 75N92020D00006/HL/NHLBI NIH HHS/United States
- UL1 TR001873/TR/NCATS NIH HHS/United States
- R01 AG023629/AG/NIA NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- N01 HC095165/HL/NHLBI NIH HHS/United States
- N01 HC095164/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
