Repurposed drugs and their combinations prevent morbidity-inducing dermonecrosis caused by diverse cytotoxic snake venoms
- PMID: 38097534
- PMCID: PMC10721902
- DOI: 10.1038/s41467-023-43510-w
Repurposed drugs and their combinations prevent morbidity-inducing dermonecrosis caused by diverse cytotoxic snake venoms
Abstract
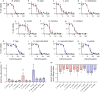
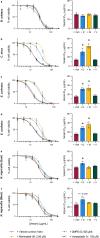
Morbidity from snakebite envenoming affects approximately 400,000 people annually. Tissue damage at the bite-site often leaves victims with catastrophic life-long injuries and is largely untreatable by current antivenoms. Repurposed small molecule drugs that inhibit specific snake venom toxins show considerable promise for tackling this neglected tropical disease. Using human skin cell assays as an initial model for snakebite-induced dermonecrosis, we show that the drugs 2,3-dimercapto-1-propanesulfonic acid (DMPS), marimastat, and varespladib, alone or in combination, inhibit the cytotoxicity of a broad range of medically important snake venoms. Thereafter, using preclinical mouse models of dermonecrosis, we demonstrate that the dual therapeutic combinations of DMPS or marimastat with varespladib significantly inhibit the dermonecrotic activity of geographically distinct and medically important snake venoms, even when the drug combinations are delivered one hour after envenoming. These findings strongly support the future translation of repurposed drug combinations as broad-spectrum therapeutics for preventing morbidity caused by snakebite.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

