The deubiquitinating enzyme USP44 suppresses hepatocellular carcinoma progression by inhibiting Hedgehog signaling and PDL1 expression
- PMID: 38097536
- PMCID: PMC10721641
- DOI: 10.1038/s41419-023-06358-y
The deubiquitinating enzyme USP44 suppresses hepatocellular carcinoma progression by inhibiting Hedgehog signaling and PDL1 expression
Abstract
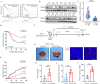
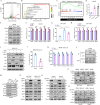
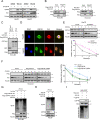
Hepatocellular carcinoma (HCC) is one of the deadliest malignancies in the world. Research into the key genes that maintain the malignant behavior of cancer cells is crucial for the treatment of HCC. Here, we identified ubiquitin-specific peptidase 44 (USP44), a member of the deubiquitinase family, as a novel regulator of HCC progression. The tumor suppressive function of USP44 was evaluated in a series of in vitro and in vivo experiments. Through quantitative proteomics examination, we demonstrated that USP44 inhibits HCC PDL1 expression by downregulating the Hedgehog (Hh) signaling pathway. Mechanistically, we found that USP44 directly interacts with Itch, an E3 ligase involved in Hh signaling, and promotes the deubiquitination and stabilization of Itch. These events result in the proteasomal degradation of Gli1 and subsequent inactivation of Hh signaling, which ultimately suppresses PDL1 expression and the progression of HCC. Furthermore, the HCC tissue microarray was analyzed by immunohistochemistry to evaluate the pathological relevance of the USP44/Itch/Gli1/PDL1 axis. Finally, the Gli1 inhibitor GANT61 was found to act in synergy with anti-PDL1 therapy. Overall, USP44 can act as a suppressive gene in HCC by modulating Hh signaling, and co-inhibition of Gli1 and PDL1 might be an effective novel combination strategy for treating HCC patients.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

