Csu pili dependent biofilm formation and virulence of Acinetobacter baumannii
- PMID: 38097635
- PMCID: PMC10721868
- DOI: 10.1038/s41522-023-00465-6
Csu pili dependent biofilm formation and virulence of Acinetobacter baumannii
Abstract
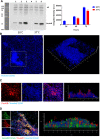
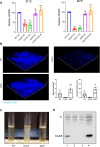
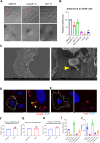
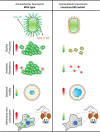
Acinetobacter baumannii has emerged as one of the most common extensive drug-resistant nosocomial bacterial pathogens. Not only can the bacteria survive in hospital settings for long periods, but they are also able to resist adverse conditions. However, underlying regulatory mechanisms that allow A. baumannii to cope with these conditions and mediate its virulence are poorly understood. Here, we show that bi-stable expression of the Csu pili, along with the production of poly-N-acetyl glucosamine, regulates the formation of Mountain-like biofilm-patches on glass surfaces to protect bacteria from the bactericidal effect of colistin. Csu pilus assembly is found to be an essential component of mature biofilms formed on glass surfaces and of pellicles. By using several microscopic techniques, we show that clinical isolates of A. baumannii carrying abundant Csu pili mediate adherence to epithelial cells. In addition, Csu pili suppressed surface-associated motility but enhanced colonization of bacteria into the lungs, spleen, and liver in a mouse model of systemic infection. The screening of c-di-GMP metabolizing protein mutants of A. baumannii 17978 for the capability to adhere to epithelial cells led us to identify GGDEF/EAL protein AIS_2337, here denoted PdeB, as a major regulator of Csu pili-mediated virulence and biofilm formation. Moreover, PdeB was found to be involved in the type IV pili-regulated robustness of surface-associated motility. Our findings suggest that the Csu pilus is not only a functional component of mature A. baumannii biofilms but also a major virulence factor promoting the initiation of disease progression by mediating bacterial adherence to epithelial cells.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

