APOBEC-1 deletion enhances cisplatin-induced acute kidney injury
- PMID: 38097707
- PMCID: PMC10721635
- DOI: 10.1038/s41598-023-49575-3
APOBEC-1 deletion enhances cisplatin-induced acute kidney injury
Abstract
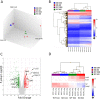
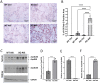
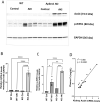
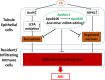
Cisplatin (CP) induces acute kidney injury (AKI) whereby proximal tubules undergo regulated necrosis. Repair is almost complete after a single dose. We now demonstrate a role for Apolipoprotein B mRNA editing enzyme, catalytic polypeptide 1 (Apobec-1) that is prominently expressed at the interface between acute and chronic kidney injury (CKD), in the recovery from AKI. Apobec-1 knockout (KO) mice exhibited greater mortality than in wild type (WT) and more severe AKI in both CP- and unilateral ischemia reperfusion (IR) with nephrectomy. Specifically, plasma creatinine (pCr) 2.6 ± 0.70 mg/dL for KO, n = 10 and 0.16 ± 0.02 for WT, n = 6, p < 0.0001 in CP model and 1.34 ± 0.22 mg/dL vs 0.75 ± 0.06, n = 5, p < 0.05 in IR model. The kidneys of Apobec-1 KO mice showed increased necrosis, increased expression of KIM-1, NGAL, RIPK1, ASCL4 and increased lipid accumulation compared to WT kidneys (p < 0.01). Neutrophils and activated T cells were both increased, while macrophages were reduced in kidneys of Apobec-1 KO animals. Overexpression of Apobec-1 in mouse proximal tubule cells protected against CP-induced cytotoxicity. These findings suggest that Apobec-1 mediates critical pro-survival responses to renal injury and increasing Apobec-1 expression could be an effective strategy to mitigate AKI.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
DGM is named a co-inventor on pending patent “Methods and Systems for Diagnosis of Acute Interstitial Nephritis” and the founder of Predict AIN, LLC, a medical diagnostics company.
Figures




References
MeSH terms
Substances
Grants and funding
- RC1DK086465/GF/NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- P30 DK079310/DK/NIDDK NIH HHS/United States
- RC1 DK086402/DK/NIDDK NIH HHS/United States
- K23DK117065/DK/NIDDK NIH HHS/United States
- R01 DK119437/DK/NIDDK NIH HHS/United States
- R01 DK126815/DK/NIDDK NIH HHS/United States
- RC1 DK086465/DK/NIDDK NIH HHS/United States
- DK-119437/GF/NIH HHS/United States
- R01 DK128087/DK/NIDDK NIH HHS/United States
- R01 DK081037/DK/NIDDK NIH HHS/United States
- P30 DK052574/DK/NIDDK NIH HHS/United States
- K23 DK117065/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

