Iron accumulation drives fibrosis, senescence and the senescence-associated secretory phenotype
- PMID: 38097808
- PMCID: PMC10730403
- DOI: 10.1038/s42255-023-00928-2
Iron accumulation drives fibrosis, senescence and the senescence-associated secretory phenotype
Abstract
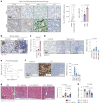
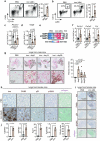
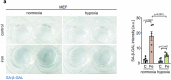
Fibrogenesis is part of a normal protective response to tissue injury that can become irreversible and progressive, leading to fatal diseases. Senescent cells are a main driver of fibrotic diseases through their secretome, known as senescence-associated secretory phenotype (SASP). Here, we report that cellular senescence, and multiple types of fibrotic diseases in mice and humans are characterized by the accumulation of iron. We show that vascular and hemolytic injuries are efficient in triggering iron accumulation, which in turn can cause senescence and promote fibrosis. Notably, we find that senescent cells persistently accumulate iron, even when the surge of extracellular iron has subdued. Indeed, under normal conditions of extracellular iron, cells exposed to different types of senescence-inducing insults accumulate abundant ferritin-bound iron, mostly within lysosomes, and present high levels of labile iron, which fuels the generation of reactive oxygen species and the SASP. Finally, we demonstrate that detection of iron by magnetic resonance imaging might allow non-invasive assessment of fibrotic burden in the kidneys of mice and in patients with renal fibrosis. Our findings suggest that iron accumulation plays a central role in senescence and fibrosis, even when the initiating events may be independent of iron, and identify iron metabolism as a potential therapeutic target for senescence-associated diseases.
© 2023. The Author(s).
Conflict of interest statement
M. Serrano is a shareholder of Senolytic Therapeutics, Life Biosciences, Rejuveron Senescence Therapeutics and Altos Labs and is an advisor to Rejuveron Senescence Therapeutics and Altos Labs. M. Serrano has been a consultant, until the end of 2022, for Rejuveron Senescence Therapeutics and Altos Labs. The funders had no role in the study design, data collection and analysis, decision to publish or manuscript preparation. The remaining authors declare no competing interests.
Figures












References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

