Restoration of sensory feedback from the foot and reduction of phantom limb pain via closed-loop spinal cord stimulation
- PMID: 38097809
- PMCID: PMC11404213
- DOI: 10.1038/s41551-023-01153-8
Restoration of sensory feedback from the foot and reduction of phantom limb pain via closed-loop spinal cord stimulation
Erratum in
-
Publisher Correction: Restoration of sensory feedback from the foot and reduction of phantom limb pain via closed-loop spinal cord stimulation.Nat Biomed Eng. 2024 Nov;8(11):1502. doi: 10.1038/s41551-023-01175-2. Nat Biomed Eng. 2024. PMID: 38155296 No abstract available.
Abstract
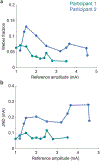
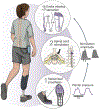
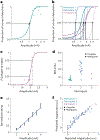
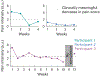
Restoring somatosensory feedback in individuals with lower-limb amputations would reduce the risk of falls and alleviate phantom limb pain. Here we show, in three individuals with transtibial amputation (one traumatic and two owing to diabetic peripheral neuropathy), that sensations from the missing foot, with control over their location and intensity, can be evoked via lateral lumbosacral spinal cord stimulation with commercially available electrodes and by modulating the intensity of stimulation in real time on the basis of signals from a wireless pressure-sensitive shoe insole. The restored somatosensation via closed-loop stimulation improved balance control (with a 19-point improvement in the composite score of the Sensory Organization Test in one individual) and gait stability (with a 5-point improvement in the Functional Gait Assessment in one individual). And over the implantation period of the stimulation leads, the three individuals experienced a clinically meaningful decrease in phantom limb pain (with an average reduction of nearly 70% on a visual analogue scale). Our findings support the further clinical assessment of lower-limb neuroprostheses providing somatosensory feedback.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Figures





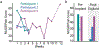
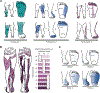


References
-
- Sexton AT & Fleming LL Lower extremity amputations. In Medical Management of the Surgical Patient: A Textbook of Perioperative Medicine (eds Walker HK, Lubin MF, Spell NO, Smith RB & Dodson TF) 741–743 (Cambridge University Press, 2006).
-
- Viseux FJF The sensory role of the sole of the foot: review and update on clinical perspectives. Neurophysiol. Clin 50, 55–68 (2020). - PubMed
-
- Petersen BA, Nanivadekar AC, Chandrasekaran S & Fisher LE Phantom limb pain: peripheral neuromodulatory and neuroprosthetic approaches to treatment. Muscle Nerve 59, 154–167 (2019). - PubMed
-
- Kuffler DP Origins of phantom limb pain. Mol. Neurobiol 55, 60–69 (2018). - PubMed
MeSH terms
Grants and funding
- F30 HD098794/HD/NICHD NIH HHS/United States
- F30HD098794/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- P1FRP3_188027/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- UH3NS100541/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- UH3 NS100541/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

