Biofilm formation: mechanistic insights and therapeutic targets
- PMID: 38097907
- PMCID: PMC10721784
- DOI: 10.1186/s43556-023-00164-w
Biofilm formation: mechanistic insights and therapeutic targets
Abstract
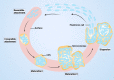
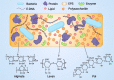
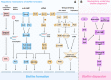
Biofilms are complex multicellular communities formed by bacteria, and their extracellular polymeric substances are observed as surface-attached or non-surface-attached aggregates. Many types of bacterial species found in living hosts or environments can form biofilms. These include pathogenic bacteria such as Pseudomonas, which can act as persistent infectious hosts and are responsible for a wide range of chronic diseases as well as the emergence of antibiotic resistance, thereby making them difficult to eliminate. Pseudomonas aeruginosa has emerged as a model organism for studying biofilm formation. In addition, other Pseudomonas utilize biofilm formation in plant colonization and environmental persistence. Biofilms are effective in aiding bacterial colonization, enhancing bacterial resistance to antimicrobial substances and host immune responses, and facilitating cell‒cell signalling exchanges between community bacteria. The lack of antibiotics targeting biofilms in the drug discovery process indicates the need to design new biofilm inhibitors as antimicrobial drugs using various strategies and targeting different stages of biofilm formation. Growing strategies that have been developed to combat biofilm formation include targeting bacterial enzymes, as well as those involved in the quorum sensing and adhesion pathways. In this review, with Pseudomonas as the primary subject of study, we review and discuss the mechanisms of bacterial biofilm formation and current therapeutic approaches, emphasizing the clinical issues associated with biofilm infections and focusing on current and emerging antibiotic biofilm strategies.
Keywords: Anti-biofilm drugs; Antibiofilm therapeutic strategy; Biofilm; Pseudomonas.
© 2023. The Author(s).
Conflict of interest statement
The author declares no competing financial interests.
Figures

Similar articles
-
Development of Antibiofilm Therapeutics Strategies to Overcome Antimicrobial Drug Resistance.Microorganisms. 2022 Jan 27;10(2):303. doi: 10.3390/microorganisms10020303. Microorganisms. 2022. PMID: 35208758 Free PMC article. Review.
-
Three lines of defense: A multifunctional coating with anti-adhesion, bacteria-killing and anti-quorum sensing properties for preventing biofilm formation of Pseudomonas aeruginosa.Acta Biomater. 2022 Oct 1;151:254-263. doi: 10.1016/j.actbio.2022.08.008. Epub 2022 Aug 9. Acta Biomater. 2022. PMID: 35961522
-
Heterogeneity in surface sensing suggests a division of labor in Pseudomonas aeruginosa populations.Elife. 2019 Jun 10;8:e45084. doi: 10.7554/eLife.45084. Elife. 2019. PMID: 31180327 Free PMC article.
-
Evolving biofilm inhibition and eradication in clinical settings through plant-based antibiofilm agents.Phytomedicine. 2023 Oct;119:154973. doi: 10.1016/j.phymed.2023.154973. Epub 2023 Jul 17. Phytomedicine. 2023. PMID: 37499434 Review.
-
Recent perspectives on the molecular basis of biofilm formation by Pseudomonas aeruginosa and approaches for treatment and biofilm dispersal.Folia Microbiol (Praha). 2018 Jul;63(4):413-432. doi: 10.1007/s12223-018-0585-4. Epub 2018 Jan 19. Folia Microbiol (Praha). 2018. PMID: 29352409 Review.
Cited by
-
Targeting Bacterial Biofilms on Medical Implants: Current and Emerging Approaches.Antibiotics (Basel). 2025 Aug 6;14(8):802. doi: 10.3390/antibiotics14080802. Antibiotics (Basel). 2025. PMID: 40867996 Free PMC article. Review.
-
Multidrug resistance in Pseudomonas aeruginosa: genetic control mechanisms and therapeutic advances.Mol Biomed. 2024 Nov 27;5(1):62. doi: 10.1186/s43556-024-00221-y. Mol Biomed. 2024. PMID: 39592545 Free PMC article. Review.
-
Synergistic Effects of Pyrrosia lingua Caffeoylquinic Acid Compounds with Levofloxacin Against Uropathogenic Escherichia coli: Insights from Molecular Dynamics Simulations, Antibiofilm, and Antimicrobial Assessments.Molecules. 2024 Nov 30;29(23):5679. doi: 10.3390/molecules29235679. Molecules. 2024. PMID: 39683837 Free PMC article.
-
Functional hydrogels promote chronic infectious wound healing by re-rousing macrophage M1 and inducing bacterial copper-like death.Mater Today Bio. 2025 Feb 13;31:101571. doi: 10.1016/j.mtbio.2025.101571. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40051527 Free PMC article. Review.
-
Molecular Mechanisms of Virulence Regulation in Staphylococcus aureus: A Journey into Reconstitutive Biochemistry.Acc Chem Res. 2025 May 20;58(10):1657-1669. doi: 10.1021/acs.accounts.5c00117. Epub 2025 May 7. Acc Chem Res. 2025. PMID: 40331756
References
Publication types
Grants and funding
- ZYYC21001/1·3·5 Project of Excellent Development of Discipline of West China Hospital of Sichuan University
- 2022SCUH0029/the "Zero to One" Innovation Research Project of Sichuan University
- 82072999 82273320/National Natural Science Foundation of China
- 82241049 81922042 82172285/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
