Neoadjuvant nivolumab plus chemotherapy in resectable non-small-cell lung cancer in Japanese patients from CheckMate 816
- PMID: 38098261
- PMCID: PMC10859619
- DOI: 10.1111/cas.16030
Neoadjuvant nivolumab plus chemotherapy in resectable non-small-cell lung cancer in Japanese patients from CheckMate 816
Abstract
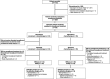
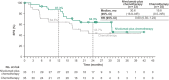
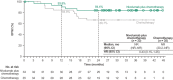
In the open-label, phase III CheckMate 816 study (NCT02998528), neoadjuvant nivolumab plus chemotherapy demonstrated statistically significant improvements in event-free survival (EFS) and pathological complete response (pCR) versus chemotherapy alone in patients with resectable non-small-cell lung cancer (NSCLC). Here we report efficacy and safety outcomes in the Japanese subpopulation. Patients with stage IB-IIIA, resectable NSCLC were randomized 1:1 to nivolumab plus chemotherapy or chemotherapy alone for three cycles before undergoing definitive surgery within 6 weeks of completing neoadjuvant treatment. The primary end-points (EFS and pCR) and safety were assessed in patients enrolled at 16 centers in Japan. Of the Japanese patients randomized, 93.9% (31/33) in the nivolumab plus chemotherapy arm and 82.9% (29/35) in the chemotherapy arm underwent surgery. At 21.5 months' minimum follow-up, median EFS was 30.6 months (95% confidence interval [CI], 16.8-not reached [NR]) with nivolumab plus chemotherapy versus 19.6 months (95% CI, 8.5-NR) with chemotherapy; hazard ratio, 0.60 (95% CI, 0.30-1.24). The pCR rate was 30.3% (95% CI, 15.6-48.7) versus 5.7% (95% CI, 0.7-19.2), respectively; odds ratio, 7.17 (95% CI, 1.44-35.85). Grade 3/4 treatment-related adverse events were reported in 59.4% versus 42.9% of patients, respectively, with no new safety signals identified. Neoadjuvant nivolumab plus chemotherapy resulted in longer EFS and a higher pCR rate versus chemotherapy alone in Japanese patients, consistent with findings in the global population. These data support nivolumab plus chemotherapy as a neoadjuvant treatment option in Japanese patients with resectable NSCLC.
Keywords: Japanese; minimally invasive surgical procedure; neoadjuvant therapy; nivolumab; non-small-cell lung carcinoma.
© 2023 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
T. Mitsudomi is an Associate Editor of
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

