PPARG dysregulation as a potential molecular target in adrenal Cushing's syndrome
- PMID: 38098864
- PMCID: PMC10720662
- DOI: 10.3389/fendo.2023.1265794
PPARG dysregulation as a potential molecular target in adrenal Cushing's syndrome
Abstract
Background: We performed a transcriptomic analysis of adrenal signaling pathways in various forms of endogenous Cushing's syndrome (CS) to define areas of dysregulated and druggable targets.
Methodology: Next-generation sequencing was performed on adrenal samples of patients with primary bilateral macronodular adrenal hyperplasia (PBMAH, n=10) and control adrenal samples (n=8). The validation groups included cortisol-producing adenoma (CPA, n=9) and samples from patients undergoing bilateral adrenalectomy for Cushing's disease (BADX-CD, n=8). In vivo findings were further characterized using three adrenocortical cell-lines (NCI-H295R, CU-ACC2, MUC1).
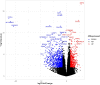
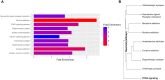
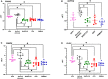
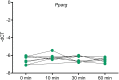
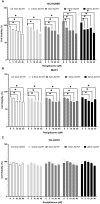
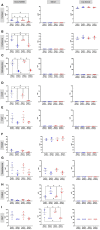
Results: Pathway mapping based on significant expression patterns identified PPARG (peroxisome proliferator-activated receptor gamma) pathway as the top hit. Quantitative PCR (QPCR) confirmed that PPARG (l2fc<-1.5) and related genes - FABP4 (l2fc<-5.5), PLIN1 (l2fc<-4.1) and ADIPOQ (l2fc<-3.3) - were significantly downregulated (p<0.005) in PBMAH. Significant downregulation of PPARG was also found in BADX-CD (l2fc<-1.9, p<0.0001) and CPA (l2fc<-1.4, p<0.0001). In vitro studies demonstrated that the PPARG activator rosiglitazone resulted in decreased cell viability in MUC1 and NCI-H295R (p<0.0001). There was also a significant reduction in the production of aldosterone, cortisol, and cortisone in NCI-H295R and in Dihydrotestosterone (DHT) in MUC1 (p<0.05), respectively.
Outcome: This therapeutic effect was independent of the actions of ACTH, postulating a promising application of PPARG activation in endogenous hypercortisolism.
Keywords: adrenocortical cell line; cortisol; hypercortisolism; primary bilateral macronodular hype; rosiglitazone; steroidome; transcriptome.
Copyright © 2023 Vetrivel, Tamburello, Oßwald, Zhang, Khan, Jung, Baker, Rainey, Nowak, Altieri, Detomas, Watts, Williams, Wielockx, Beuschlein, Reincke, Sbiera and Riester.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures




References
-
- Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol (2016) 175:G1–G34. doi: 10.1530/EJE-16-0467 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

